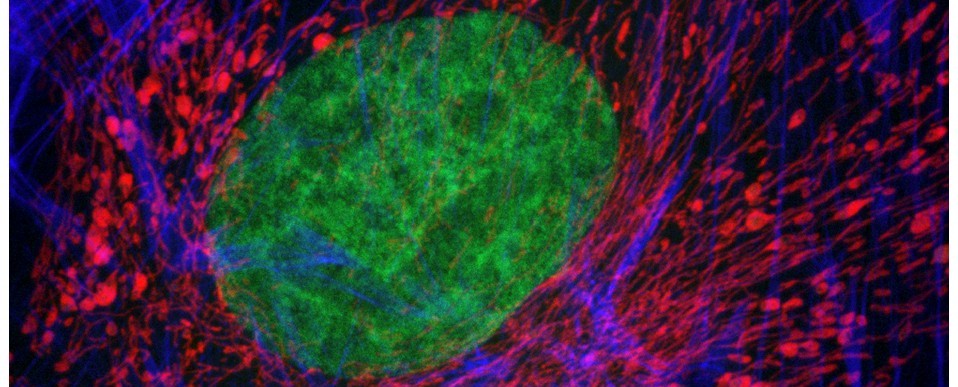
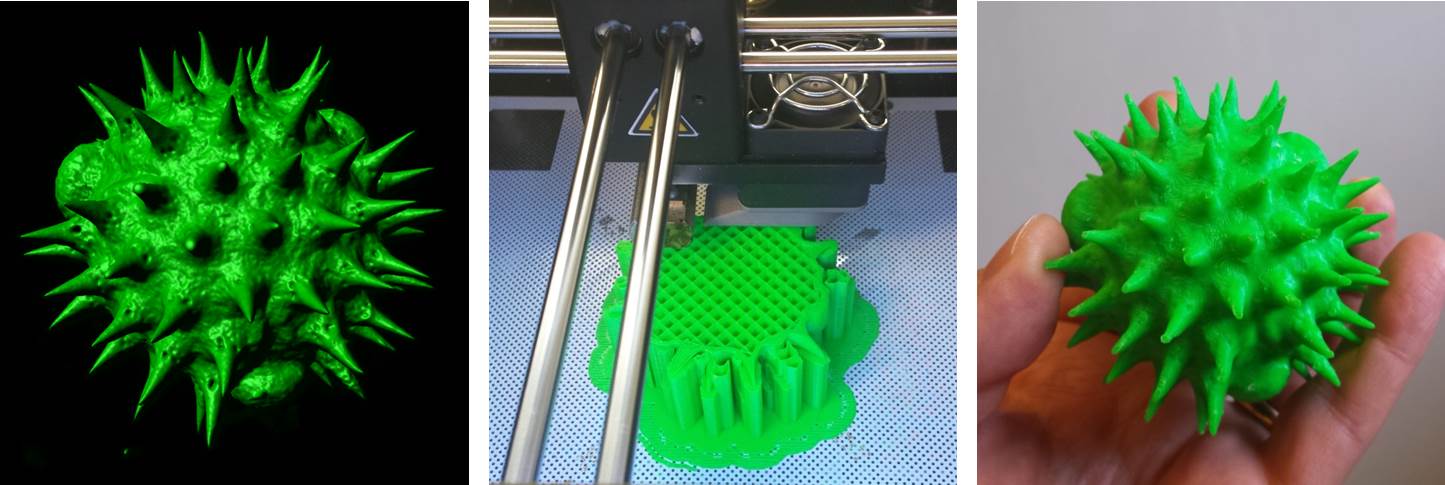
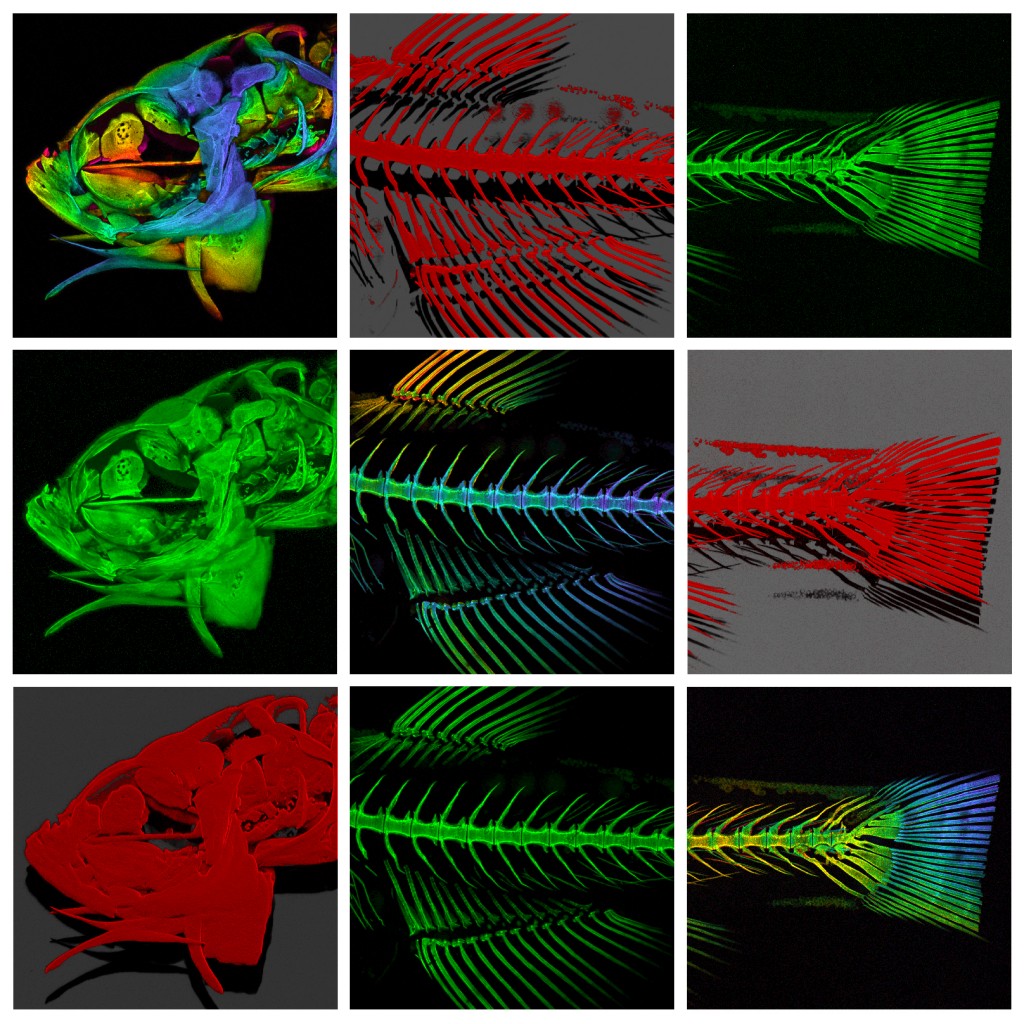
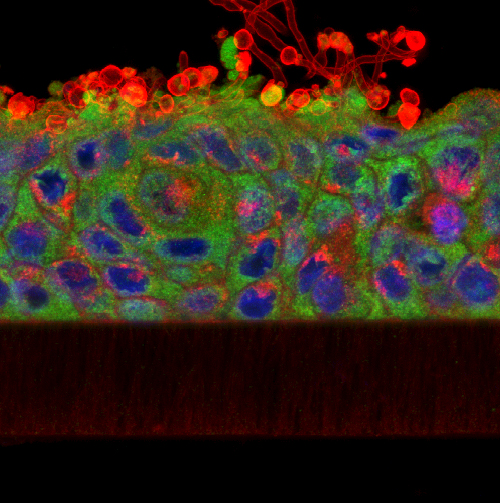
Imagine being able to generate a highly accurate, solid scale replica of the sample that you are visualising down the microscope; a perfectly-rendered pollen grain, or blood cell, or microscopic organism, but big enough to hold and examine in your hand. It would allow much better 3D conceptualisation of the sample, particularly for blind or visually-impaired individuals, and would have enormous utility in teaching and in engagement activities, and what researcher wouldn’t want a tangible, physical embodiment of their research to help explain their work (and impress their colleagues) at scientific meetings? Sounds like the stuff of science fiction doesn’t it? Well, not any more. Thanks to 3D printing technology (and the help of Dr Simon Scofield‘s lab) we have started taking volume datasets from the confocal microscope out of the virtual world and making them a reality. If you would be interested in generating a highly accurate scale model of your favourite biological sample (or would simply like to handle a giant pollen grain!) then please feel free to get in touch.
AJH
Further reading:
- Perry, I., Szeto, J-Y., Isaacs, M.D., Gealy, E.C., Rose, R., Scofield, S., Watson, P.D., Hayes, A.J. (2017) Production of 3D printed scale models from microscope volume datasets for use in STEM education. EMS Engineering Science Journal. 1 (1): 002.