You may remember one of our blogs from 2015 about a virtual histology slidebox in development by the Bioimaging Research Hub? If not, link here. Well, I’m very pleased to report that we’ve made considerable progress since then.
The resource has now been moved from its humble beginnings (a Rasberry Pi/raid drive set-up) to a new PC server based within the Bioimaging Research Hub. The database has also been developed significantly through mySQL which allows efficient management of the image metadata via a web interface, allowing the images to be sorted, filtered and navigated online.
The image collection has now been expanded significantly; thus, in addition to the original histopathology collection (which contained approximately 400 digitised sections of normal and pathological tissues), we now have two new additional sections on cell biology and parasitology.
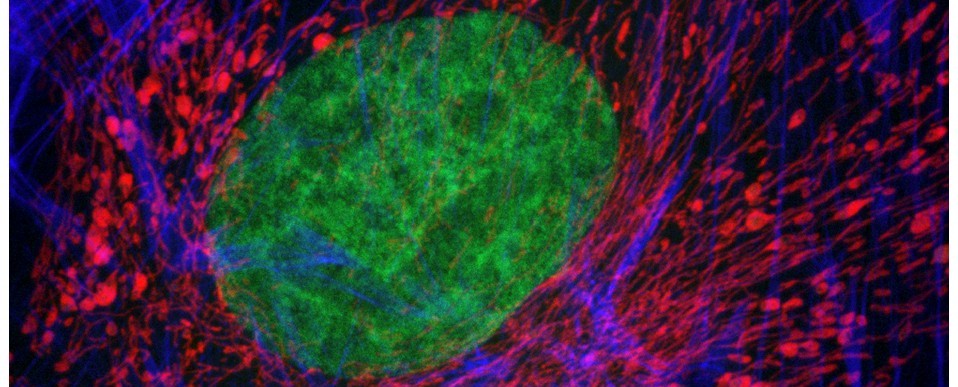
The cell biology section contains both zoomable/navigable images and interactive 3D models of intracellular structure, including major organelles, cytoplasmic inclusions and cytoskeletal components. These were all generated from confocal fluorescence datasets imaged using our Zeiss LSM880 airyscan confocal system and rendered in 3D via Bitplane Imaris. The parasitology section, meanwhile, contains over 200 new zoomable/navigable images of various parasitic species, organised phylogenetically for easy reference and sorting. As before, these images were generated using our Navigator slide scanning system.
So far, the database has been trialed for small group anatomy teaching, as well as for a number of BIOSI practical classes including Research Techniques (#BIT002), Advanced Research Techniques (#BI4002) and the Identifying Organelles (#BI2231) module. It is also utilized extensively for outreach and engagement activities within the Bioimaging hub to showcase our research capabilities.
As the database is a bespoke system that has been developed in house, there are no costly subscriptions involved. We are also uniquely positioned within the Bioimaging Hub to expand and develop the database according to the needs of the user. It therefore has enormous potential as a centralized repository for microscopical image data for teaching, research and outreach/engagement purposes.
We are planning to add additional sections on plant biology and entomology and we would welcome collaboration with any BIOSI staff who have access to the relevant slide resources and would be happy to help in curating these collections.
Ultimately, the plan is to find a permanent home for the virtual microscopy database as part of the new e-learning and assessment facility (eLEAF) within BIOSI.
Please take a look at the database here: http://vmdb.bios.cf.ac.uk/. Any feedback (+/-) would be welcome.
Thanks again to all involved so far.
AJH









![[Parameter-Settings] FileVersion = 2000 Date/Time = 0000:00:00 00:00:00 Date/Time + ms = 0000:00:00,00:00:00:000 User Name = TCS User Width = 1032 Length = 1032 Bits per Sample = 8 Used Bits per Sample = 8 Samples per Pixel = 3 ScanMode = xy Series Name = demo2.lei](http://blogs.cardiff.ac.uk/bioimaging/wp-content/uploads/sites/492/2016/09/Photobleach-1024x509.jpg)