Alzheimer’s is slowly giving up its secrets – and ‘risk genes’ are just one piece of the puzzle
3 September 2018
September is World Alzheimer’s Month – an annual international campaign to raise awareness and challenge the stigma that surrounds dementia. Here, Anna Burt (School of Medicine) tells us how researchers are using genetic information to piece together our understanding of the disease.
Although the causes of Alzheimer’s disease remain a mystery, genetic research is now providing clues about how the disease develops. We know that rare genetic mutations can cause early-onset Alzheimer’s, however, both genetic and environmental factors are involved in the more common, late-onset form of the disease. By collecting information on the genetic make-up of thousands of people, scientists in our group, and others, have identified nearly 30 gene variants that are more common in the disease.
The function of many of these “risk genes” in the brain is unknown, but they appear to cluster by biological function, giving us greater insight into the mechanisms involved in Alzheimer’s. One of the biological functions implicated in Alzheimer’s from these genetic findings is the transport of material into the cell, known as endocytosis. This occurs when material cannot passively cross the cell membrane, the cell buds inwards to capture the cargo in a small fluid-filled sac.
Our research group is investigating what these endocytic genes do in the brain. Using cells grown in a dish, we can manipulate the proteins they express and measure changes in the uptake of material by the cell. This helps us to understand what happens when these genes are impaired in Alzheimer’s.
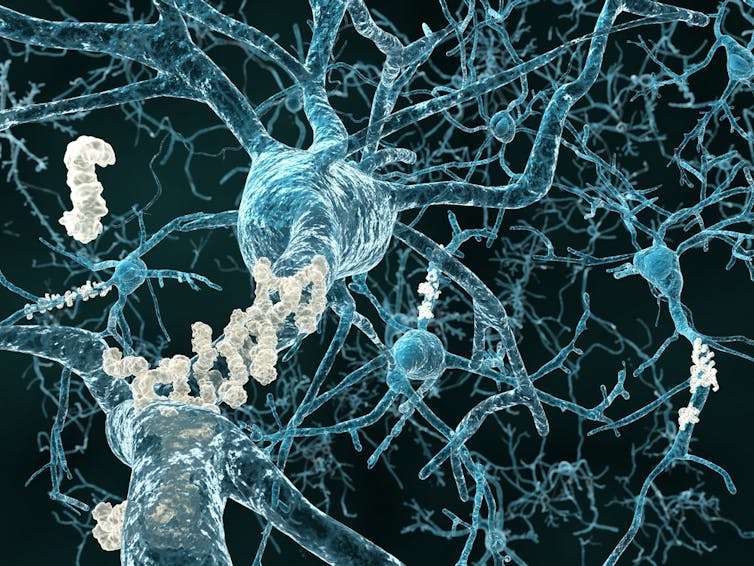
Endocytosis is a universally important cell function, all cells need to eat and drink. It is also responsible for many other vital tasks including communication, transport and cleaning up waste products, such as beta-amyloid. This is a protein produced in the healthy brain which is normally broken down and eliminated. In Alzheimer’s disease, however, it is thought that an imbalance of beta-amyloid production and its removal from the brain causes a build-up and the formation of sticky clumps, known as plaques, which are toxic to neurons.
Brain housekeeping
Mopping up beta-amyloid is one of the functions carried out by microglia, the immune cells of the brain. They are the first responders when an intruder enters the brain. They use their endocytic abilities to engulf and destroy waste material and infectious agents. In Alzheimer’s, this function is key as they consume beta-amyloid, breaking it down through their internal waste-disposal system.
Beta-amyloid can also be removed via the 650km of blood vessels throughout the human brain. The endothelial cells lining the vessels form a tight barrier between the blood and the brain. This stops toxic agents but allows nutrients to enter and waste products to escape. Beta-amyloid is removed from the brain by a special form of endocytosis that involves it binding to a receptor on the surface of endothelial cells, like a lock and key. This triggers its internalisation and it is carried across the cell and deposited in the blood, preventing a build-up in the brain.
Juan Gaertner/Shutterstock.com
How does beta-amyloid get there in the first place?
The precise function of beta-amyloid is still unclear, but it is likely that it plays a role in normal brain physiology, only becoming a problem when present in excess. It is produced by the breakdown of the amyloid precursor protein (APP), found on the surface of cells in the brain.
APP can be broken down in two different ways, only one of which produces beta-amyloid. The enzyme responsible for this pathway resides inside the cell, so APP must undergo endocytosis in order to be broken into beta-amyloid fragments.
Part of our research involves measuring the amount of beta-amyloid and other fragments produced by cells as a result of APP breakdown. Comparing these between healthy cells and those where Alzheimer’s risk genes have been manipulated allows us to understand the involvement of these genes in beta-amyloid production. Here, again, endocytosis appears as a crucial player in the potential development of Alzheimer’s disease.
This illustrates just three examples of how endocytosis is important for brain health and how a fault in any of these could be a contributory factor to Alzheimer’s development. Indeed, many other biological processes have been implicated from genetic studies and these are unlikely to be mutually exclusive. With the addition of both lifestyle and environmental risk factors, Alzheimer’s is highly complex. Nonetheless, by understanding the various mechanisms involved, we can begin to identify potential targets for treatment. Much like a jigsaw puzzle, we are using genetic information as corner pieces to build up a clearer picture of the disease as a whole.![]()
This article was originally published on The Conversation. Read the original article.
Comments
1 comment
Comments are closed.
- June 2024
- March 2024
- February 2024
- November 2023
- September 2023
- June 2023
- May 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- July 2022
- May 2022
- April 2022
- January 2022
- December 2021
- November 2021
- August 2021
- July 2021
- June 2021
- February 2021
- January 2021
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- Biosciences
- Careers
- Conferences
- Development
- Doctoral Academy Champions
- Doctoral Academy team
- Events
- Facilities
- Funding
- Humanities
- Internships
- Introduction
- Mental Health
- PGR Journeys
- Politics
- Public Engagement
- Research
- Sciences
- Social Sciences
- Staff
- STEM
- Success Stories
- Top tips
- Training
- Uncategorized
- Wellbeing
- Working from home
Very nice explanation, it helped me a lot