 1. Development of Novel Selenium Electrophiles: Selenium-based reagents have found broad application in organic chemistry and are frequently used in synthesis. We have developed easily accessible and highly effective selenium-based reagents of type 1 – 3 and successfully used them in many different reactions. These reagents allow for the chemo-, regio– and stereoselective introduction of new functional groups into complex organic molecules under particularly mild reaction conditions. The continued need for efficient stereoselective transformations led us to develop chiral selenium-based reagents. We have used these reagents in key steps for a number of natural product syntheses allowing for high levels of stereocontrol. Furthermore, we have demonstrated that polymer-supported versions of these reagents (2) can be used with comparable efficiency. Selenium-based reagents bear a high potential for the improvement of known reactions not only from an environmental and pharmaceutical point of view, but also as interesting reagents for the development of completely new synthetic transformations and as potential ligands in catalytic reactions.
1. Development of Novel Selenium Electrophiles: Selenium-based reagents have found broad application in organic chemistry and are frequently used in synthesis. We have developed easily accessible and highly effective selenium-based reagents of type 1 – 3 and successfully used them in many different reactions. These reagents allow for the chemo-, regio– and stereoselective introduction of new functional groups into complex organic molecules under particularly mild reaction conditions. The continued need for efficient stereoselective transformations led us to develop chiral selenium-based reagents. We have used these reagents in key steps for a number of natural product syntheses allowing for high levels of stereocontrol. Furthermore, we have demonstrated that polymer-supported versions of these reagents (2) can be used with comparable efficiency. Selenium-based reagents bear a high potential for the improvement of known reactions not only from an environmental and pharmaceutical point of view, but also as interesting reagents for the development of completely new synthetic transformations and as potential ligands in catalytic reactions.
 2. Synthesis of Natural Products and other Applications: We have been investigating chiral selenium electrophiles of type 1 in addition reactions to non-activated alkenes. The addition products are formed in up to 96% de and are versatile building blocks, which we have used successfully for the enantioselective synthesis of different natural products. We completed the synthesis of the furofuran lignan Phrymarolin I 4 (isolated from Phryma Leptostachya), but also simple alkaloids such as Salsolidine 5 have been prepared. Stereoselective selenenylation is the key-step in their relatively short total syntheses. The mechanisms of the reaction and the transition states involved have been intensively investigated experimentally and by various computational methods. We have also developed polymer-bound reagents such as 2, which are recyclable after an addition reaction and cleavage of the product from the reagent. With reagents of this type, we are able to generate enantiomerically enriched acetals such as 6 and determine their absolute configuration. This is very noteworthy as our chiral selenium electrophiles allow stereoselective access to such compounds for the first time. Nucleophile-selective selenocyclisations to give compounds like 7 have recently revealed further insight into the conditions for selective cyclizations. Several of the selenium-based reagents can easily be synthesised in large quantities and are highly efficient.
2. Synthesis of Natural Products and other Applications: We have been investigating chiral selenium electrophiles of type 1 in addition reactions to non-activated alkenes. The addition products are formed in up to 96% de and are versatile building blocks, which we have used successfully for the enantioselective synthesis of different natural products. We completed the synthesis of the furofuran lignan Phrymarolin I 4 (isolated from Phryma Leptostachya), but also simple alkaloids such as Salsolidine 5 have been prepared. Stereoselective selenenylation is the key-step in their relatively short total syntheses. The mechanisms of the reaction and the transition states involved have been intensively investigated experimentally and by various computational methods. We have also developed polymer-bound reagents such as 2, which are recyclable after an addition reaction and cleavage of the product from the reagent. With reagents of this type, we are able to generate enantiomerically enriched acetals such as 6 and determine their absolute configuration. This is very noteworthy as our chiral selenium electrophiles allow stereoselective access to such compounds for the first time. Nucleophile-selective selenocyclisations to give compounds like 7 have recently revealed further insight into the conditions for selective cyclizations. Several of the selenium-based reagents can easily be synthesised in large quantities and are highly efficient.
 3. Selenium Reagents as Catalysts: As the use of stoichiometric amounts of seleium-based reagents should be avoided, we also develop new strategies towards catalytic reactions using chiral selenium-based reagents. We have discovered that nitrogen-based chiral diselenides such as 3 are excellent pro-catalysts for stereoselective dialkylzinc additions to aldehydes; the secondary alcohols are obtained in up to 98% yield and with excellent selectivities (up to 98% ee). Recently, we developed an addition – elimination sequence with catalytic amounts of a diselenide to synthesize butenolides such as 8.
3. Selenium Reagents as Catalysts: As the use of stoichiometric amounts of seleium-based reagents should be avoided, we also develop new strategies towards catalytic reactions using chiral selenium-based reagents. We have discovered that nitrogen-based chiral diselenides such as 3 are excellent pro-catalysts for stereoselective dialkylzinc additions to aldehydes; the secondary alcohols are obtained in up to 98% yield and with excellent selectivities (up to 98% ee). Recently, we developed an addition – elimination sequence with catalytic amounts of a diselenide to synthesize butenolides such as 8.
 4. Selenium Compounds as Glutathione Peroxidase Mimics: Various diselenides 9 have been examined with respect to their glutathione peroxidase-like activities as an index of their antioxidant properties. The glutathione oxidase activities of some of these compounds have also been investigated regarding their pro-oxidant properties (GOx-activities) which can be used as an index of cell toxicity.
4. Selenium Compounds as Glutathione Peroxidase Mimics: Various diselenides 9 have been examined with respect to their glutathione peroxidase-like activities as an index of their antioxidant properties. The glutathione oxidase activities of some of these compounds have also been investigated regarding their pro-oxidant properties (GOx-activities) which can be used as an index of cell toxicity.
5. Iodine Reagents in Synthesis: Halocyclisations are important reactions for the generation of functionalised heterocycles. Despite the numerous applications of halocyclisations and the importance of stereoselective synthesis for the preparation of biologically active compounds among other applications, stereoselective, reagent-controlled halocyclisations are scarce. We are developing halonium ions in a chiral environment as the requisite reagents for such reactions. We have synthesised chiral amine-iodine monochloride complexes and have used them successfully in asymmetric iodolactonisations of carboxylic acids 10 leading to iodolactones 11 in up to 50% ee.
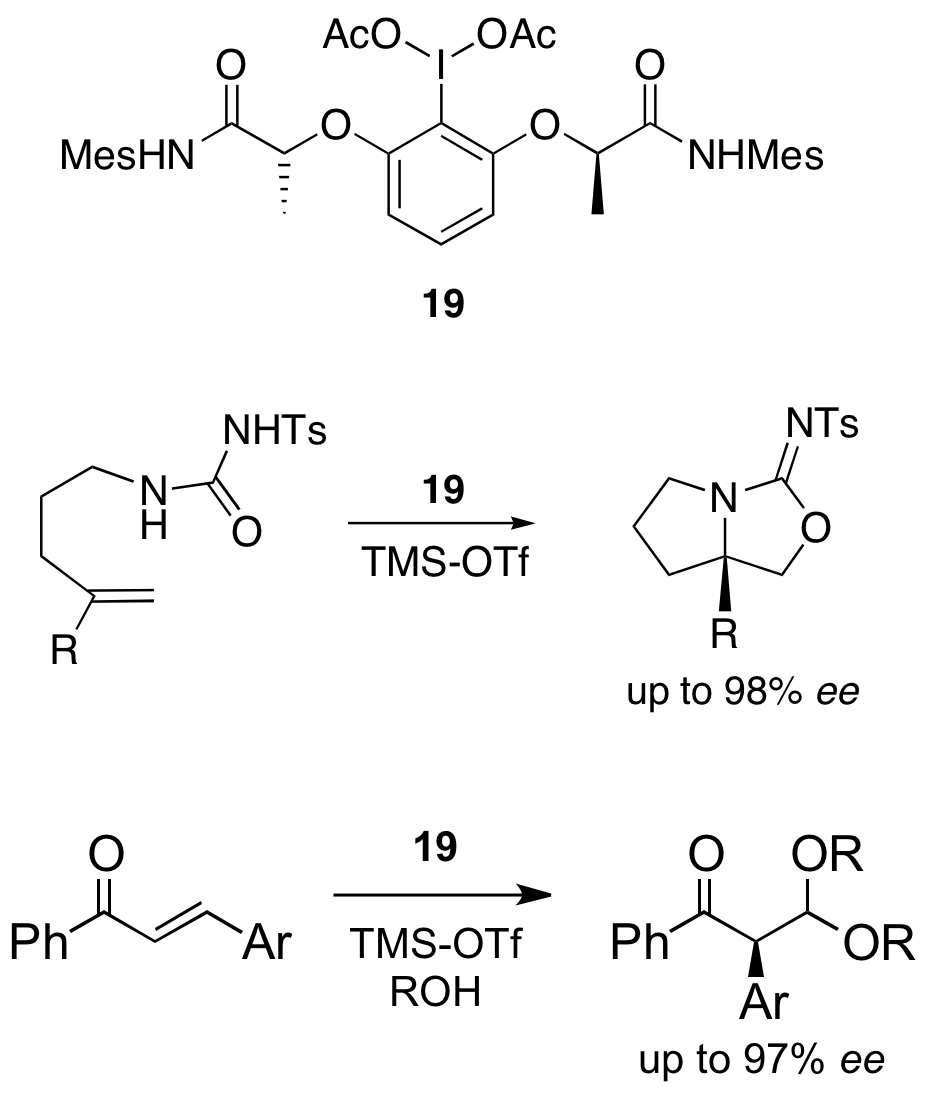 6. Hypervalent Iodine Reagents: Hypervalent iodine compounds are popular reagents being very efficient oxidants, which tolerate a wide range of functional groups. In addition to oxidations, the electrophilic properties of these reagents are used for attractive functionalisations. We have synthesised several stable chiral hypervalent iodine compounds of type 12 which allowed us to carry out the first enantioselective reactions using hypervalent iodine reagents. Alkenes can be ditosylated with up to 65% ee (13) and ketones can be stereoselectively functionalised in the a-position (13) even with catalytic amounts of iodine reagents in combination with a stoichiometric oxidant. We have also published an easy and fast access to (difluoroiodo) derivatives such as 15 avoiding the use of elemental fluorine, which are capable of fluorinating various substrates under mild conditions. Fluorinated compounds of type 16 are accessible with these reagents in a seleno-Pummerer type reaction. Recently, we have developed highly reactive reagents such as FIBX 17 and 18 for oxidative transformations. The hypervalent compound 18 allows for the first time a direct oxidation of sulfides to sulfoximines in good yields. New cyclisations using the C2 symmetrical hypervalent iodine reagent 19 are leading to very high selectivities (up to 98% ee) in the bicyclic aminohydroxylated products. Also the first stereoselective rearrangements (up to 97% ee) with chiral hypervalent iodine reagents have been published recently.
6. Hypervalent Iodine Reagents: Hypervalent iodine compounds are popular reagents being very efficient oxidants, which tolerate a wide range of functional groups. In addition to oxidations, the electrophilic properties of these reagents are used for attractive functionalisations. We have synthesised several stable chiral hypervalent iodine compounds of type 12 which allowed us to carry out the first enantioselective reactions using hypervalent iodine reagents. Alkenes can be ditosylated with up to 65% ee (13) and ketones can be stereoselectively functionalised in the a-position (13) even with catalytic amounts of iodine reagents in combination with a stoichiometric oxidant. We have also published an easy and fast access to (difluoroiodo) derivatives such as 15 avoiding the use of elemental fluorine, which are capable of fluorinating various substrates under mild conditions. Fluorinated compounds of type 16 are accessible with these reagents in a seleno-Pummerer type reaction. Recently, we have developed highly reactive reagents such as FIBX 17 and 18 for oxidative transformations. The hypervalent compound 18 allows for the first time a direct oxidation of sulfides to sulfoximines in good yields. New cyclisations using the C2 symmetrical hypervalent iodine reagent 19 are leading to very high selectivities (up to 98% ee) in the bicyclic aminohydroxylated products. Also the first stereoselective rearrangements (up to 97% ee) with chiral hypervalent iodine reagents have been published recently.
 7. Microreactor Chemistry: The introduction of more general platforms to perform reactions under continuous flow rather than in batch mode has led to improvements regarding safety and sustainability.The miniaturisation of chemical processes using chip-based microreactors exhibits significant advantages. A high surface-to-volume ratio, short diffusion distances, fast and efficient heat dissipation and mass transfer enable novel and diverse applications. For a simple biphasic hydrolysis, we could show that the application of various reaction conditions in microreactors using segmented flow can dramatically increase the reaction rate. The combination of two processes (A -> B, C -> D) on one reactor would result in convergent synthesis of product E. A tandem metathesis / Heck-reaction served as an example that even homogeneous reactions can be accelerated by segmentation in microreactors. Techniques for the determination of reaction rates using microreactors have been developed. A dramatic reduction in processing time and potential for manufacturing scale-out is possible.
7. Microreactor Chemistry: The introduction of more general platforms to perform reactions under continuous flow rather than in batch mode has led to improvements regarding safety and sustainability.The miniaturisation of chemical processes using chip-based microreactors exhibits significant advantages. A high surface-to-volume ratio, short diffusion distances, fast and efficient heat dissipation and mass transfer enable novel and diverse applications. For a simple biphasic hydrolysis, we could show that the application of various reaction conditions in microreactors using segmented flow can dramatically increase the reaction rate. The combination of two processes (A -> B, C -> D) on one reactor would result in convergent synthesis of product E. A tandem metathesis / Heck-reaction served as an example that even homogeneous reactions can be accelerated by segmentation in microreactors. Techniques for the determination of reaction rates using microreactors have been developed. A dramatic reduction in processing time and potential for manufacturing scale-out is possible.
8. Synthesis of PET Tracer Molecules: The combination of microreactor technology and the synthesis of PET tracer molecules is currently investigated together with Eckert & Ziegler AG, Berlin, Germany. A general platform to perform small-scale reactions under sterile conditions is being developed and tested. Several PET tracer molecules such as FDG (using segmented flow in microreactors) and 18F-DOPA (using polymer-bound diaryliodonium compounds as versatile precursors) are currently being synthesized and, through access to radioactive iostopes, will be available for clinical tests.
