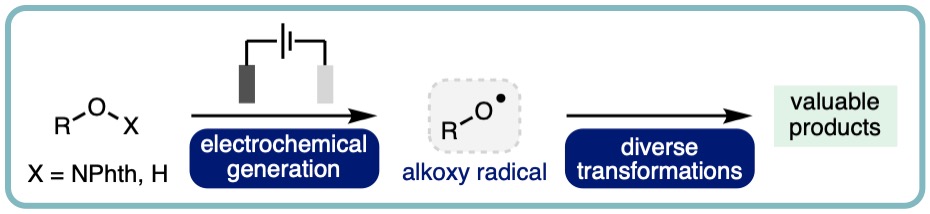
Electrochemical Generation and Utilization of Alkoxy Radicals
This highlight summarises electrochemical approaches for the generation and utilization of alkoxy radicals, predominantly focusing on recent advances (2012-present). The application of electrochemically generated alkoxy radicals in a diverse range of transformations is described, including discussion on reaction mechanisms, scope and limitations, in addition to highlighting future challenges in this burgeoning area of sustainable synthesis. (Chem. Commun., 2023, 59, 3655-3664.) [link] [Part of themed collection: Chemical Communications Hot Articles 2023]
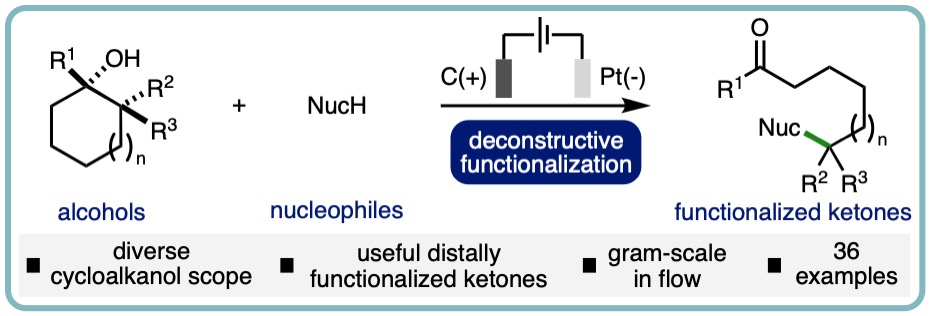
Deconstructive Functionalization of Unstrained Cycloalkanols via Electrochemically-Generated Aromatic Radical Cations
Herein we report an electrochemical approach for the deconstructive functionalization of cycloalkanols, where various alcohols, carboxylic acids, and N-heterocycles are employed as nucleophiles. The method has been demonstrated across a broad range of cycloalkanol substrates, including various ring sizes and substituents, to access useful remotely functionalized ketone products (36 examples). The method was demonstrated on gram-scale via single pass continuous flow, which exhibited increased productivity in relation to the batch process. (Org. Lett., 2023, 25, 1486-1490). [link]
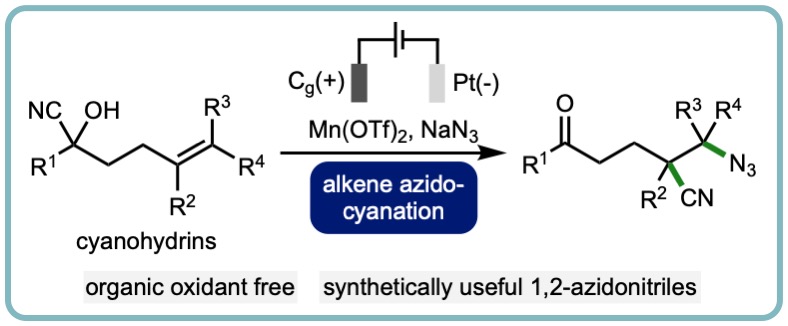
Electrochemical Alkene Azidocyanation via 1,4-Nitrile Migration
An electrochemical method for the azidocyanation of alkenes via 1,4-nitrile migration has been developed. This organic oxidant free method is applicable across various alkene containing cyanohydrins, and provides access to a broad range of synthetically useful 1,2-azidonitriles (28 examples). This methodology was extended to an electrochemical alkene sulfonylcyanation procedure, as well as to access a trifunctionalized hexanenitrile from a malononitrile starting material. The orthogonal derivatization of the products was also demonstrated through chemoselective transformations. (Chem. Commun., 2022, 58, 8658-8662.) [link]
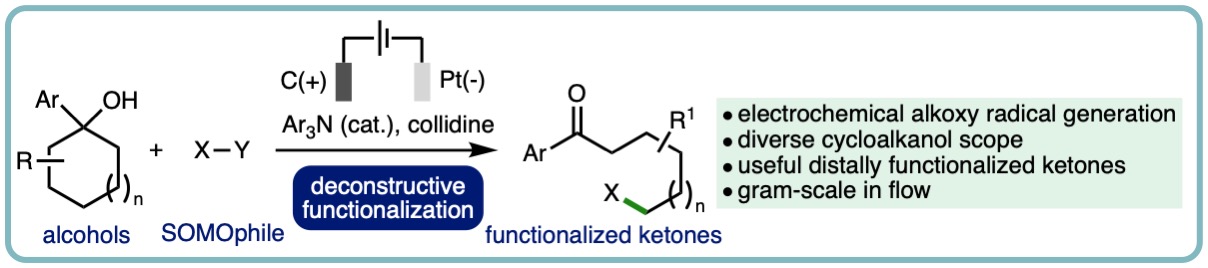
Electrochemical Deconstructive Functionalization of Cycloalkanols via Alkoxy Radicals Enabled by Proton-Coupled Electron Transfer
Herein, we report a new electrochemical method for alkoxy radical generation from alcohols using a Proton-Coupled Electron Transfer (PCET) approach, showcased via the deconstructive functionalization of cycloalkanols. The electrochemical method is applicable across a diverse array of substituted cycloalkanols, accessing a broad range of synthetically useful distally func-tionalized ketones. The orthogonal derivatization of the products has been demonstrated through chemoselective transfor-mations, and the electrochemical process has been performed on gram scale in continuous single-pass flow. (Org. Lett., 2022, 24, 3890-3895.) [link]
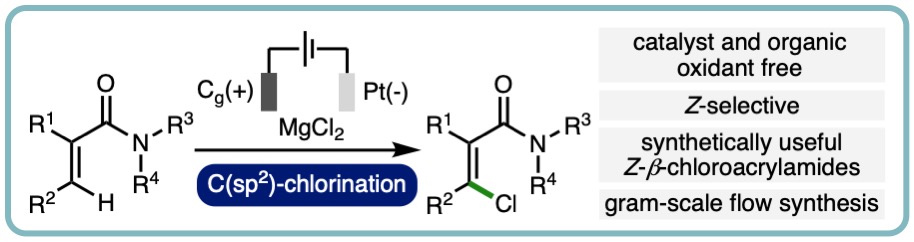
Electrochemical oxidative Z-selective C(sp2)-H chlorination of acrylamides
An electrochemical method for the oxidative Z-selective C(sp2)–H chlorination of acrylamides has been developed. This catalyst and organic oxidant free method is applicable across various substituted tertiary acrylamides, and provides access to a broad range of synthetically useful Z–b-chloroacrylamides in good yields (22 examples, 73% average yield). The orthogonal derivatization of the products was demonstrated through chemoselective transformations and the electrochemical process was performed on gram scale in flow. (Chem. Commun., 2021, 57, 12643-12646). [link] [Invited contribution to the 2021 Emerging Investigators themed collection]
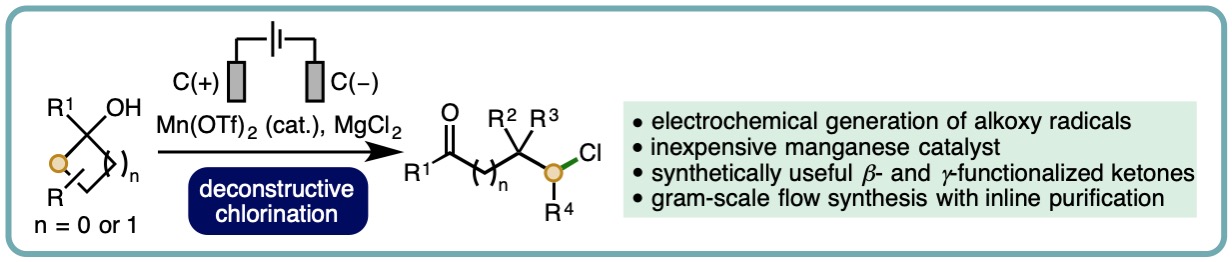
Manganese-Catalyzed Electrochemical Deconstructive Chlorination of Cycloalkanols via Alkoxy Radicals
Alkoxy radicals are highly transient species that exhibit diverse reactivity, including hydrogen atom transfer, addition to pi-systems and beta-scission processes. The generation of alkoxy radicals directly from aliphatic alcohols is challenging, partly due to the high dissociation energy of RO−H bonds (~105 kcal/mol).
A manganese-catalyzed electrochemical deconstructive chlorination of cycloalkanols has been developed. This electrochemical method provides access to alkoxy radicals from alcohols and exhibits a broad substrate scope, with various cyclopropanols and cyclobutanols converted into synthetically useful beta- and gamma-chlorinated ketones (40 examples). Furthermore, the combination of recirculating flow electrochemistry and continuous inline purification was employed to access products on gram scale (Org. Lett., 2019, 21, 9241-9246). [link]