Q-Pulse Conference November 2019
4 December 2019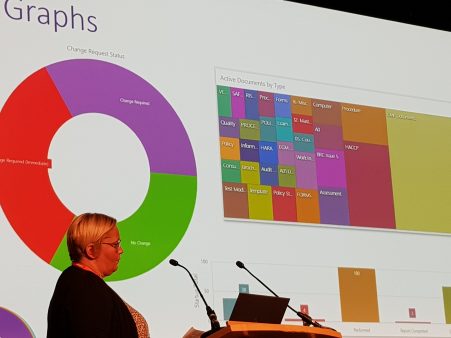
Background
Last week I attended the Q-Pulse conference at the Vox conference centre in Birmingham along with Cardiff University colleagues from Central Biotechnology Services (CBS) and the Wales Research and Diagnostic Positron Emission Tomography Imaging Centre (PETIC). It was an opportunity to explore how other organisations use Q-Pulse and to gather information on other software offered by Ideagen.
The conference itself was attended by a mix of people from healthcare and aviation, all using Q-Pulse for their Quality Management System.
Version 7.0 of Q-Pulse became available in July 2019 – its installation has been scheduled by Cardiff University IT for some time now – we hope to see progress with this by the end of 2019. Version 7.0 is a web-based version, so staff accessing Q-Pulse outside of the office should have access to the same functionality within the database as when using it on their desktop.
Dashboards
Version 7.0 also introduces 3 dashboards:
• Audit – overview
• CAPA
• Documents outstanding acknowledgements (department)
The dashboards can have an active role in data-driven continual improvement and data can be displayed as a donut, bar, or tree chart.
Ideagen plan to make version 7.1 available in December 2019 and Version 8.0 will become available in 2020.
Currently at the Centre for Trials Research we only use the document, audit corrective and preventive action (CAPA), people and training modules. We do not utilise the incidents and occurrences module, or the asset module. It was interesting to explore the uses of these additional modules and reflect on how useful they could be within the Centre for Trials Research.
Incidents and Occurrences Module
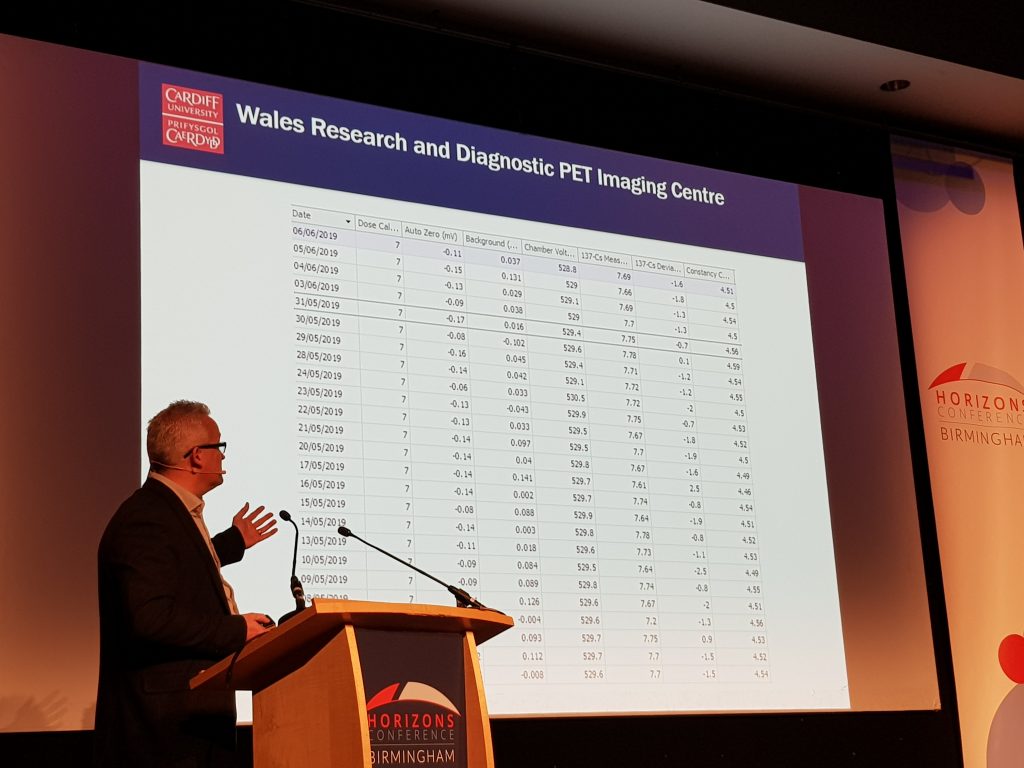
Professor Christopher Marshall, from PETIC, Cardiff University, presented a session on how PETIC use the incidents and occurrences modules to design and use forms which are employed in their sterile clean rooms. This has removed the need for paper and has increased the sterility of their clean room environment. PETIC have a longer-term goal to become paperless by utilising Q-Pulse for all their forms in the next year.
The occurrence module is a data capture module, where you can design forms – any paper forms can be transferred into an electronic form in the occurrence module. Q-Pulse has a powerful reporting tool, which allows you to aggregate the information entered in the forms to produce reports. The occurrence module can be accessed via the Q-Pulse iPad app. This has inspired me to investigate the use of the occurrence module for some of our QA paper forms, such as the Standard Operating Procedure (SOP) deviation form, SOP exemption form, impact assessment form, and process document activation checklist. I hope to make some progress with this in 2020 and would encourage others to think about the use of this module for simple data capture in their own areas of work.

Asset Module
The asset module provides you with the ability to assign any number of actions to an asset record, and from there, automatically monitor these across a complete inventory. The module also highlights pending and outstanding actions and communicates them to the relevant people until they have been addressed. This ensures exposure to unregulated equipment is minimised. This module may be of use to the Technical Solutions group within the Centre for Trials Research to help monitor computer systems and software. I’m sure there are other innovative ways this module could be used by other groups within the Centre for Trials Research too.
In addition to customer experiences presented, the conference included keynote speakers (Steve Backshall, Baroness Manningham-Buller and Nick Fry). They discussed managing and handling change during their careers, as well as their respective leadership experiences and the accompanying challenges they faced. It added an interesting dimension to the conference, which I thoroughly enjoyed and hope to attend again in the future.
Claire Johnson
Head of Quality Assurance and Regulatory Affairs
Centre for Trials Research
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016