The Role of Streptavidin and its variants in Catalysis by Biotinylated Secondary Amines
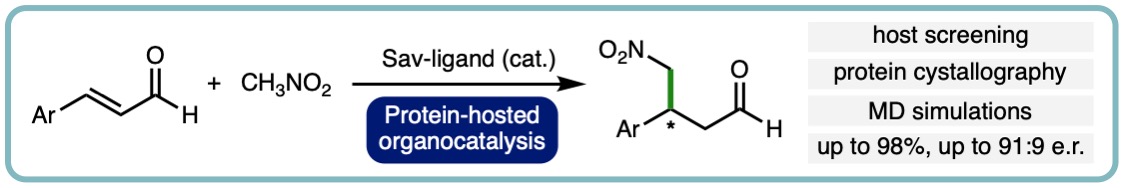
Here, we combine the use of host screening, protein crystallography and QM/MM molecular dynamics simulations to investigate how protein structure affects iminium catalysis by biotinylated secondary amines in a model 1,4 conjugate addition reaction. Monomeric streptavidin (M-Sav) lacks a quaternary structure and the solvent-exposed reaction site resulted in poor product conversion for the model reaction with low enantio- and regioselectivity. These parameters were much improved when the tetrameric host T-Sav was used; indeed, residues at the symmetrical subunit interface were proven to be critical for catalysis through mutagenesis study. Use of QM/MM simulations and the asymmetric dimeric variant D-Sav revealed that both Lys121 residues which located in the hosting and neighboring subunits play critical role in dictating the stereoselectivity and reactivity. Lastly, we viewed that the D-Sav template, though offering a lower conversion than that of the symmetric tetrameric counterpart, is likely a better starting point for future protein engineering because each surrounding residue within the asymmetric scaffold can be refined for secondary amine catalysis. (Org. Biomol. Chem., 2021, 19, 10424-10431) [link].
Transfer Hydrogenations Catalyzed by Steptavidin-hosted Seccondary Amine Organocatalyst
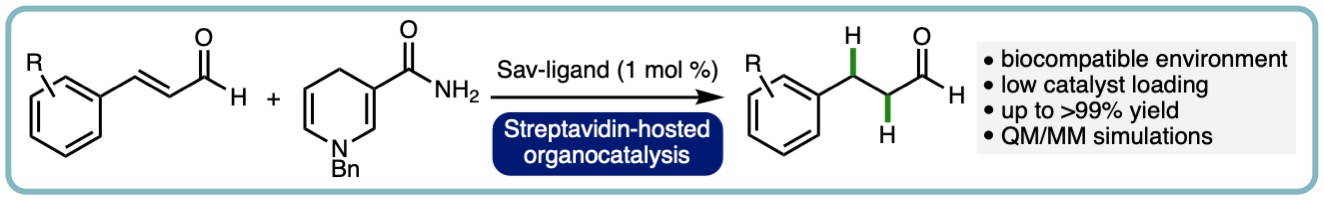
Here, the streptavidin-biotin technology was applied to enable organocatalytic transfer hydrogenation. By introducing a biotin-tethered pyrrolidine to the tetrameric streptavidin (T-Sav), the resulting hybrid catalyst was able to mediate hydride transfer from dihydro-benzylnicotinamide (BNAH) to α,β-unsaturated aldehydes. Hydrogenation of cinnamaldehyde and some of its aryl-substituted analogues was found to be nearly quantitative. Kinetic measurements revealed that the T-Sav:1 assembly possesses enzyme-like behavior, whereas isotope effect analysis, performed by QM/MM simulations, illustrated the step of hydride transfer is at least partially rate-limiting. These results have proven the concept that T-Sav can be used to host secondary amine-catalyzed transfer hydrogenations. (Chem. Commun., 2021, 57, 1919-1922) [link].
Streptavidin-hosted Organocatalytic Aldol Addition
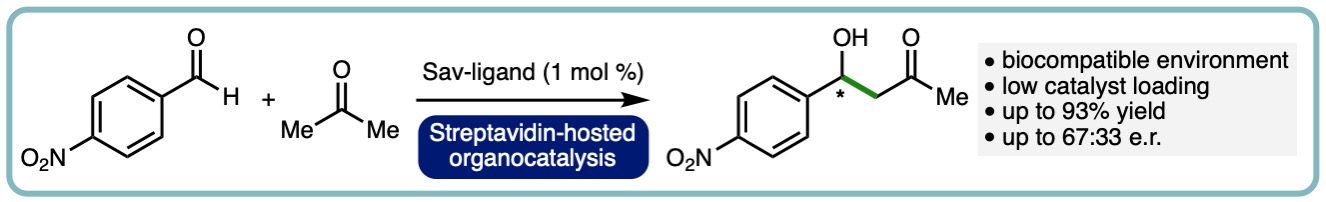
In this report, the streptavidin-biotin technology has been applied to enable organocatalytic aldol condensation. By attaching pyrrolidine to the valeric motif of biotin and introducing to streptavidin (Sav), a protein-based organocatalytic system was created, and the aldol condensation of acetone with p-nitrobenzaldehyde was tested. The conversion of substrate to product can be as high as 93%. Although the observed enantioselectivity was only moderate (33:67 er), further protein engineering efforts can be included to improve the selectivity. These results have proven the concept that Sav can be used to host stereoselective aldol condensation. (Molecules, 2020, 25, 2457) [link]. [Invited contribution to the Hybrid Catalysts for Asymmetric Catalysis Special Issue]
Reactivity and Selectivity of Iminium Organocatalysis Improved by a Protein Host
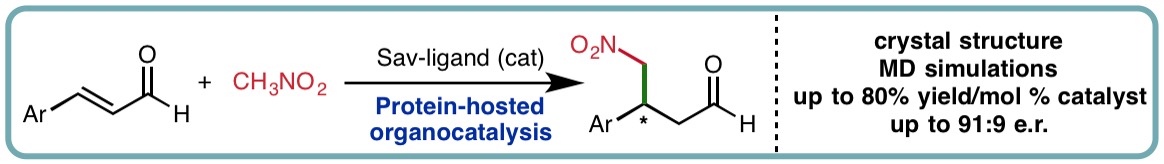
There has been growing interest in operating organocatalysis within a supramolecular system as means of controlling reaction reactivity and stereoselectivity. In a collaborative research effort led by Dr Louis Luk, a protein is used as host for iminium catalysis; a pyrrolidine moiety is covalently linked to biotin, introduces to the protein host streptavidin and tested for organocatalytic activity (Angew. Chem. Int. Ed., 2018, 57, 12478-12482) [link]. Whereas in traditional systems, stereoselectivity is largely controlled by the substituents added to the organocatalyst, enantiomeric enrichment by the reported supramolecular system is completely controlled by the host. Also, the yield of the model reaction increases over 10-fold when streptavidin is included. A 1.1-Å crystal structure of the protein-catalyst complex and molecular simulations of a key intermediate reveal the chiral scaffold surrounding the organocatalytic reaction site. This work illustrates that proteins can be an excellent supramolecular host for driving stereoselective secondary amine organocatalysis.