Spotlight on: The PATHOS Study
5 August 2020
PATHOS is a Phase III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for human papillomavirus (HPV)-positive oropharyngeal cancer.
The aim of the study is to tailor treatment for participants with HPV-positive oropharyngeal cancer to reduce side-effects, particularly swallowing problems, which have a major impact on quality of life.
We are interested to see if long term toxicity, can be reduced in patients receiving either lower dose radiotherapy (Group B) or no chemotherapy (Group C), whilst proving that cure rates can be maintained.
Recruitment
The PATHOS study team are working closely with local centres to ensure that the latest guidelines for surgery and radiotherapy / chemotherapy treatment in a COVID environment are applied as practically as possible for all participants on trial. Please feel free to contact your local PATHOS team if you have any questions that you would like to direct to the PATHOS study team.
Our recruitment target is 1,100 patients and we currently have over 400 participants enrolled. Together with your support, we are confident that we will meet this target.
We have 34 sites in the United Kingdom and international sites open in Florida and Australia. We hope to open 30 additional sites across Europe over the next few months.
Recruitment continues until October 2022 and follow-up visits will continue until 2026.
The results will be analysed and published in 2027. You will be able to access any publications and study results with the help of your study team. No confidential information will appear in any of the results or publications.
The data from the study is reviewed every 6 months by our Independent Data Monitoring Committee (IDMC) to make sure that all the patients are safe. The committee had their last meeting in April 2020 and they were very happy with the quality of the study and recommended that the study continues.
The Speech and Language Therapists are an integral part of the PATHOS study, and they are currently in the process of analysing the Videofluoroscopies (VFs).
We also collect several blood and tissue samples before treatment and at approximately 4 weeks, 6 months, 12 months, 18 months and 2 years following treatment. These blood and tissue samples will be used for future research projects, which will allow us to learn more about HPV related oropharynx cancer, as well as identifying better ways to treat the disease.
The study is looking at long term parameters, and therefore the success of the study is directly related to how many follow-up visits we manage to collect.
Timepoints
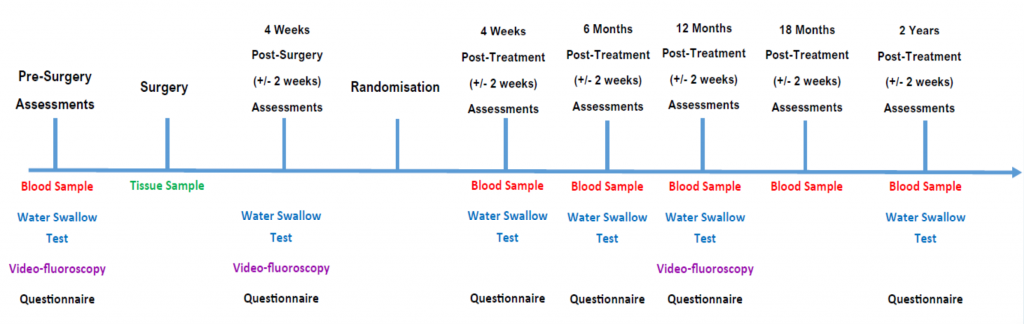
The key timepoints are:
- Pre-Surgery Assessments
- Baseline VFs and Water Swallow Tests
- 4 Weeks Post-Surgery
- 4 Weeks Post-Treatment Questionnaires (MDADI, QLQ C30 and H&N35)
- All 12 month follow-up assessments are important.
Further information
Further information is available at
Read more about the PATHOS study.
Follow on Twitter
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016