NIHR Fellowship Project Exploring Research Involving Adults Lacking Capacity To Consent – Our Public And Patient Involvement Journey
28 October 2019
Project exploring research involving adults who lack capacity to consent
For the past 3 years I have been conducting a project exploring the ethical, legal, and practical challenges that may be encountered when involving people with impaired capacity in research. The project, titled ‘Informed consent and proxy decision making in research involving adults lacking capacity: development of an intervention to support proxy informed decision making, set within ethical and legal frameworks’, was part of a National Institute for Health Research (NIHR) Fellowship funded by Health and Care Research Wales. A previous blog explained how the project came about.
Importance of including people with impaired capacity in research
Research is needed in order for improvements to be made to the health and social care provided, however, some people may be unable to decide for themselves about whether or not to take part in a research study due to a cognitive impairment. Currently, people who are unable to provide consent to take part in research are more likely to be excluded from research. But with increasing numbers of adults living longer, leading to more people with conditions like dementia, it is important that these groups are included in research.
‘Proxy’ decisions about research participation
When someone is unable to make their own decisions, a family member or friend may be asked to act as a ‘proxy’ to be involved in making decisions on their behalf. However, decisions about research are complicated, and currently there is no information or support available for proxy decision makers. This project aimed to explore the ethical and legal factors involved in proxy decisions about research, understand how families make decisions through the DECISION Study, and establish what support might help families when making such decisions. The final part of the project was to develop a support tool that is suitable to be used by families and friends when making decisions for adults lacking capacity to consent to research.
Involving patients and members of the public in research
Involving members of the public and patients in research is increasingly being seen as an essential part of how research is identified, prioritised, designed, conducted and disseminated. INVOLVE, the national advisory group, which is part of and funded by the NIHR defines public involvement as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. This includes working with research funders to prioritise research, offering advice as members of a project steering group, commenting on and developing research materials, and undertaking interviews with research participants. Patient and public involvement (PPI) includes involving patients, potential patients, carers and people who use health and social care services as well as people from organisations that represent people who use services. Public and patient involvement and engagement is also a core element in our work at the Centre for Trials Research.
Public Involvement in the ‘Proxy Consent’ Fellowship project
Active public involvement was an essential part of this research project. Prior to starting the project, two members of the public were invited to form a lay advisory panel (supported by funding from Health and Care Research Wales) to review the funding application and initial draft of the plain English summary. Once the project was funded, two additional members were recruited to join the lay advisory panel.
The project has benefitted enormously from their support from the first drafting of the research questions, through to ensuring the information provided to potential DECISION Study participants was accessible and appropriate, and the development of the intervention itself. Their lived experience as carers, family members, and advocates of people with a range of cognitive impairments has made a significant contribution to the project as a whole, and particularly during the development of the decision support intervention.
Sharing our Patient and Public Involvement journey
Members of the public get involved in research for a variety of reasons, including a desire to improve the quality of care or improve treatments either for themselves or for others with a similar condition or to give something back and help others. Involvement in research can also increase peoples’ confidence and knowledge and help them to develop new skills. An important part of involving the public is to recognise and value the contribution they make to the research project.
Towards the end of the project we thought it would be useful and interesting to ‘map out’ our PPI journey throughout the project to help identify when and how the involvement of the lay advisory group helped shape the project. Together we designed a map of the journey with additional information provided about their involvement at various stages.
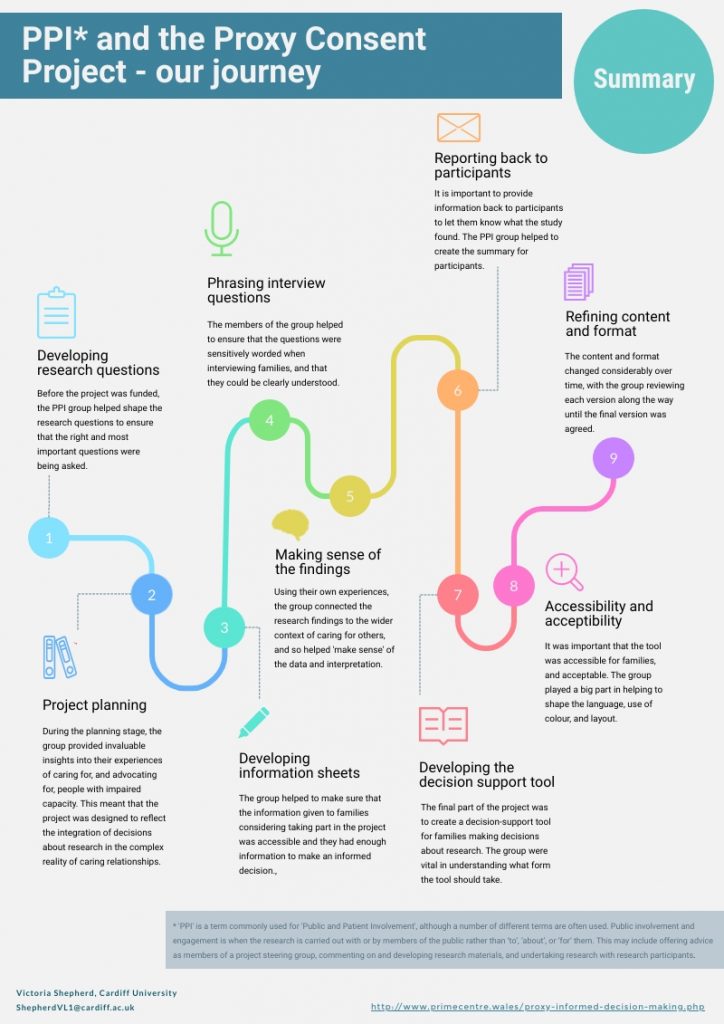
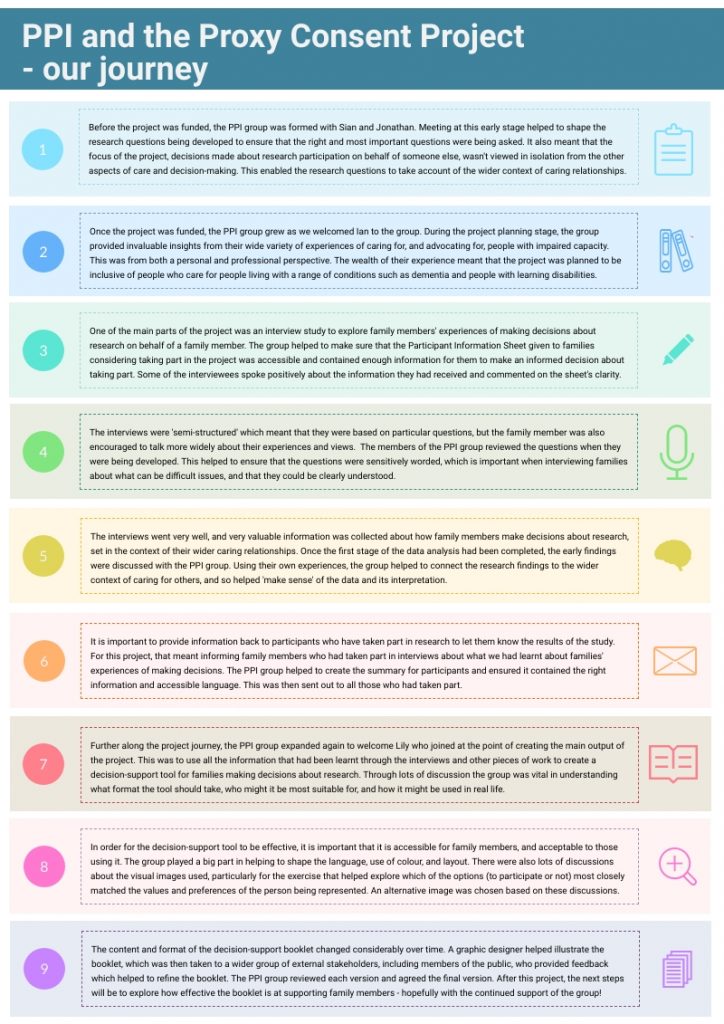
These two images then formed a poster which was presented at Health and Care Research Wales’ 2019 annual conference with the members of the lay advisory group as co-authors.
Involvement in public engagement activities
Engagement is defined by INVOLVE as the process of information and knowledge about research being provided and disseminated to audiences. Examples of engagement include science festivals, public awareness raising, and dissemination of the findings of a study to research participants or members of the public. For this project, the lay advisory group had a key role in helping draft the findings from the research, particularly those from the DECISION Study, for dissemination to the participants and wider public audiences. This included providing written summaries of the findings which were sent to all those who had taken part and posted on the Centre’s website, as well as a short animation of the findings which were uploaded to You Tube. A link to the animation was also sent to participants and shared on social media.
The benefits of public involvement and engagement
The involvement of people with experience as carers of people with impaired capacity and members of organisations that represent people who use services has led to the successful completion of the project. The results have been published in a number of peer-reviewed publications and presented both nationally and internationally. Importantly, it has led to research that is relevant to families of people with impaired capacity, to findings that are accessible to a wide range of people who may benefit from the research, and to the development of an intervention that may help families making decisions about research in the future.
Our next steps
The next step is to establish whether the intervention is an effective form of support. First, we need to establish which outcomes matter, to whom, and why. The COnSiDER project will develop a core outcome set for the evaluation of interventions to enhance proxy decisions about research on behalf of adults who lack capacity to consent. Members of the public will be involved in a Delphi study which forms part of the project to reach consensus about which outcomes should be included.
A funding application is being developed for a follow-on project to evaluate whether the intervention is effective in supporting proxy decisions about research. The lay advisory group have kindly agreed to support the development of the application and, if funded, will form a key part of the project. I know that their contribution will continue to be hugely important in the design, conduct and dissemination of that future project, and I look forward to continuing our journey.
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016