Synthesis and Reactivity of N-Allenyl Cyanamides
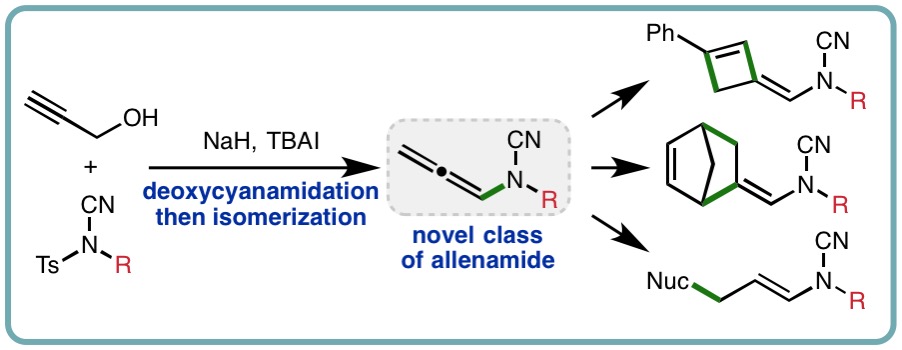
Allenamides, also referred to as N-allenyl amides, are versatile synthetic building blocks in organic chemistry. In comparison to allenamines, which require careful preparation and handling due to their susceptibility toward hydrolysis and polymerization, allenamides exhibit enhanced stability due to delocalization of the nitrogen lone pair of electrons into the electron-withdrawing carbonyl. In addition to N-acyl allenamides, alternative classes have been introduced through variation of the electron-withdrawing N-substituent, including N-phosphoryl, N-sulfonyl, and N-heteroaryl allenamides.
Continuing our collaboration with Syngenta, N-allenyl cyanamides have been accessed via a one-pot deoxycyanamidation-isomerization approach using propargyl alcohol and N-cyano-N-phenyl-p-methylbenzenesulfonamide (NCTS). The utility of this novel class of allenamide was explored through derivatization, with hydroarylation, hydroamination and cycloaddition protocols employed to access an array of cyanamide products that would be challenging to access using existing methods (Org. Lett., 2018, 20, 5282-5285) [link].
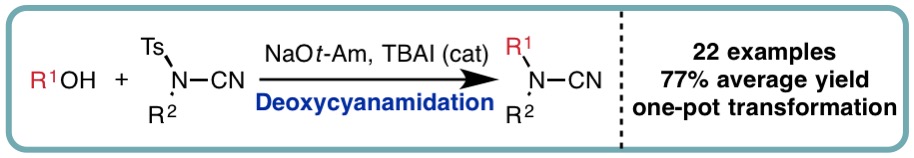
Deoxycyanamidation of Alcohols with N-Cyano-N-Phenyl-p-Methylbenzenesulfonamide (NCTS)
Electrophilic cyanation describes the reaction of C-, N-, O– and S-based nucleophiles with electrophilic nitrile sources “+CN”. Traditionally, cyanogen halides have been employed for this purpose, but their high associated toxicity has driven the development of alternative electrophilic cyanating reagents including cyanates (O-CN), thiocyanates (S-CN), cyanamides (N-CN), nitriles (C-CN) and hypervalent iodine reagents (I-CN). Among these reagents, N-cyano-N-phenyl-p-methylbenzenesulfonamide (NCTS), has been employed in a variety of N– and C-cyanation processes. However, the alternative desulfonylative reactivity pathway of NCTS remains largely under developed.
Continuing our collaboration with Syngenta, we developed the first one-pot deoxycyanamidation of alcohols using NCTS as both a sulfonyl transfer reagent and cyanamide source, accessing a diverse range of tertiary cyanamides in excellent isolated yields (Org. Lett., 2017, 19, 3835-3838) [link]. Mechanistic investigations (both experimental and computational) probed reaction intermediates, additive effects and the observed N– to O-sulfonyl transfer.
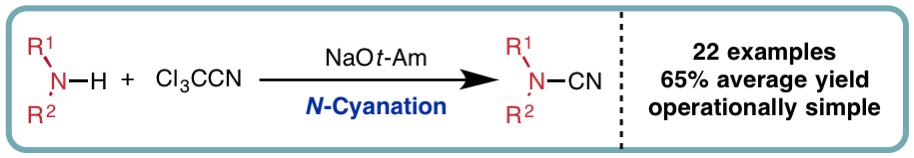
N-Cyanation of Secondary Amines using Trichloroacetonitrile
The cyanamide functionality is present in a variety of biologically active compounds including natural products, agrochemicals, and pharmaceuticals. Furthermore, cyanamides have diverse synthetic applications, serving as building blocks in the production of amidines, guanidines and various heterocyclic scaffolds. Despite their significance, the most commonly employed method for cyanamide synthesis remains the electrophilic N-cyanation of amines using highly toxic cyanogen bromide.
In a collaborative research effort with Syngenta, we developed a one-pot N-cyanation of secondary amines using trichloroacetonitrile as an inexpensive cyano source (Org. Lett., 2016, 18, 5528-5531) [link]. A diverse range of cyclic and acyclic secondary amines can be readily transformed into the corresponding cyanamides in good isolated yields, with the method successfully utilised in the final synthetic step of a biologically active rolipram-derived cyanamide. This approach exhibits distinct selectivity when compared to the use of highly toxic cyanogen bromide.