PATIENT INFORMATION SHEET
21 August 2024We are actively looking for volunteers to take part in a study on healthy immune ageing. We aim to find out if a food supplement made from wheatgerm can increase the protective immune response to a normal flu vaccine in people aged over 65 years of age. The study starts on 30th September 2024 and you are able to register your interest in joining the study by calling 02921848251 or 07480926235 or emailing the study lead Dr Lucy Jones on JonesL147@cardiff.ac.uk. You may have already made an appointment to take part. The participant information sheet below provides more information about the study.

PARTICIPANT INFORMATION SHEET
IRFLUVA – Improving the Long Term Immune Response to the Flu Vaccine
Ethics reference: IRAS 314310
We would like to invite you to take part in a research study led by Cardiff University, University of Oxford and Cardiff and the Vale University health board. It aims to learn more about the immune responses in older people. It is up to you to decide whether to take part. It is important for you to understand why the study is being done and what it will involve for you. Please take time to read the following information carefully and discuss it with others if you wish.
What is the purpose of the study?
Our immune response to viruses and bacteria (the way our body responds to infections) reduces as we age. This appears to be part of the normal aging process and can make ‘fighting’ infections more difficult. As you may already know, some infections can affect older people more seriously than people of a younger age, for example, flu and Coronavirus.
Vaccines are very important in older people. We have seen this with the UK vaccination programme for Coronavirus, where older people are made a high priority for timely vaccination. However, doctors and scientists have seen that the immune response to a vaccine can also be reduced in older people, when compared to younger people. This change in the immune response as we age is something we would like to understand more.
There is new evidence that foods containing a substance called spermidine, may be an important part of a healthy diet and important for the immune response. Spermidine is naturally found in some foods, but not in large amounts. A widely available supplement, SPDLife, made from wheatgerm, contains spermidine and can increase the amount of spermidine eaten every day. It may improve the health of the immune system in older people. We would like to find out more about how older people find taking this supplement and explore if it can improve the immune response to the flu vaccine. It is not a medication and its safety has been studied carefully.
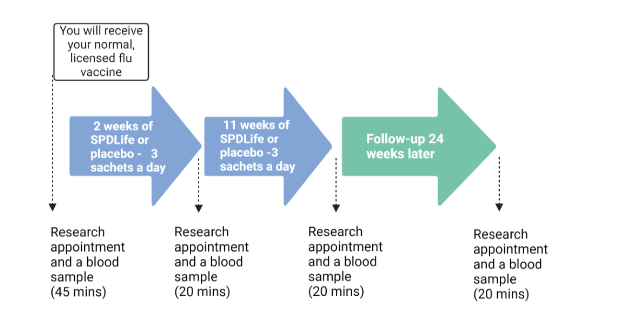
This research trial will look at if it is simple to take the supplement daily and whether older people find it acceptable. We will also explore whether it can improve the immune response in people aged more than 65 years old. If you take part, we will study the effect of this supplement on your immune response to the flu vaccine you are eligible to have every year.
We will ask you your opinion about the supplement and we will measure your antibody responses and measure the immune cells responses to the flu vaccine This will include studying your white blood cells, which help fight infection and play an essential role in successful vaccination. By analysing a sample of blood, the research will look at how your body responds to the flu vaccine and whether the response is improved by taking the supplement and ensuring you have good amount of spermidine in your diet. We hope this trial will, therefore, give us more information about whether the food supplement can be used in a larger trial in the future and to see if it can improve the immune response to the flu vaccine in older people.
What is the supplement used in this study?
The supplement is made from wheatgerm. It is a powder that dissolves in water and can be taken by mouth at home. The ‘placebo’ is also a powder that dissolves in water and can be similarly taken by mouth at home. Studies have shown the supplement does not have any serious side effects. It also contains thiamine, Zinc and Vitamin C which are important parts of a healthy diet. The placebo contains rice flour and a small amount of sucrose (a type of sugar found in food).
Why have I been chosen? We are inviting people aged 65 years or over, who are eligible to have the flu vaccine every year, to take part in this study. We are aiming to recruit a total of 80 participants to this research study. To take part you must:
|
|
|
|
You are not able to take part in the study if you have been diagnosed with one of the following conditions:
| Diabetes – Type 1 and Type 2 |
| Gluten allergy or wheat intolerance – coeliac disease |
| Cancer |
| A medical condition that reduces your immune response |
| Flu within the last 6 months |
You are also not able to take part if you:
| Had a blood/platelet or immunoglobulin treatment in the last 3 months |
| Already had your flu vaccine this year (2024) |
| Take steroids (by mouth or injection) for more than 1 week in the last 3 months |
| Have taken spermidine supplements in the last 6 months |
| Take medications that reduce your immune response |
Do I have to take part? Joining the study is entirely up to you and deciding not to take part will not affect any future standard of care. We would like you to understand why the research is being done and what it involves. One of our team will go through this information sheet with you, to help you decide whether or not you want to participate.

How do I take the supplement or placebo?
The supplement is a powder that dissolves in water. You will need to take 3 supplements per day. These can be taken at any time of day and can be taken all at one time, or spread throughout the day, depending on your preference. You can dissolve this in a small amount of water or a large glass of water, whichever works best for you. You are able to add some cordial or squash to the supplement if you prefer this.
Will I know whether I am taking SPDLife or a placebo?
During the trial you and the clinical research team will not know whether you are taking SPDLife or the placebo. At the end of the trial, the clinical research team will be able to inform you whether you took the supplement or not. You will be randomly assigned to the SPDLife group or the placebo group using a computerised clinical research trial system.
What will happen to my blood samples?
On your first and second visit to the clinical research facility, your blood will be analysed at a UK accredited medical laboratory. This sample will be labelled with your
unique study ID and date of birth. The blood tests will measure liver function, kidney function, bone health, blood sugar, iron, fats and cholesterol in the blood and a full
blood count. Before transfer of research samples to Cardiff University or Oxford University, your personal details will be removed from your blood samples so that they
will not have any identifying details on them and labelled with a unique study number. The research team will not know the results of your tests until the end of the study and
you will not be told the results of your tests. 50ml of your blood will undergo testing to study the effect of the supplement on your body, antibodies and immune cells,
including your T cells and B cells (which are important for fighting infection). We will examine how your immune cells responded to the flu vaccine with several scientific
tests. We will examine the DNA in your immune cells. If you take part all of these tests will be performed. We will not study immune cells for genetic diseases. Results of these tests will not be reported to you. If you wish to know more on this please discuss with the Principal Investigator, Dr Lucy Jones.
What will happen to my samples at the end of the study? Donated blood, immune cells and their DNA samples without your personal details on
them, will be stored at the University of Oxford and Cardiff University to ensure experiments are completed in full and may be used in future ethically approved
Studies. They will then be stored in accordance with Human Tissue Act (HTA) Regulations. Your samples may be retained at the end of this study for use in future
ethically-approved research within the UK and abroad. At this stage we do not know what the research will involve but some of it could include DNA analysis or use in the
commercial sector. Your samples will not be sold for profit and will not be used in animal research.
Will my taking part be kept confidential and how will you use my information?
We have taken very careful steps to make sure that you cannot be identified by those outside of the clinical research delivery team. Your personal information will only be accessible by the Principal Investigator, Dr Lucy Jones and the clinical research team. We will use this information to do the research or to check your records to make sure that the research is being done properly. People who do not need to know who you are will not be able to see your personal information e.g. your name or contact details. Your data will have a code number instead. We will keep all information about you safe and secure and have very strict rules and checks in place to ensure this happens.
Once we have finished the study, we will keep some of the data so we can check the results. We will write our reports in a way that no-one can work out that you took part in the study.
Will you inform my GP that I am taking part?
The research doctor will ask your permission to contact your GP to tell them you are taking part in the research study. The research team at Cardiff and the Vale University Health Board Clinical research facility (Principal Investigator and clinical research delivery team) will ask for your permission to access relevant medical records and check your vaccine record – for example, any flu or COVID vaccines you may have received or swabs for flu virus, with your consent. This information will not be shared outside of the clinical research team in a form in which you can be identified.
How will we use information about you?
We will need to use information from you and from your medical records and your GP for this research project. This information will include your: names, initials, date of birth, NHS number, contact details and information about your health and any medication you may be taking. The clinical research team will use this information to do the research or to check your records to make sure that the research is being done properly. People who do not need to know who you are will not be able to see your name or contact details. Your data will have a code number instead. We will keep all information about you safe and secure. Some of your information may be sent to Professor Katja Simon in Germany for the purpose of completing the research. They must follow our rules about keeping your information safe.
Once we have finished the study, we will keep some of the data so we can check the results. We will write our reports in a way that no-one can work out that you took part in the study.
What are your choices about how your information is used?
- You can stop being part of the study at any time, without giving a reason, but we will keep information about you that we already have.
- If you choose to stop taking part in the study, we would like to continue collecting information about your health from [central NHS records/ your hospital/ your vaccination record, your GP]. If you do not want this to happen, tell us and we will stop.
- We need to manage your records in specific ways for the research to be reliable. This means that we won’t be able to let you see or change the data we hold about you.
- If you agree to take part in this study, you will have the option to take part in future research using your data saved from this study.
Where can I find out more about how my information is used?
You can find out more about how we use your information in this research study:
- by asking one of the research team at the Clinical Research Facility
- by sending an email to the Research Lead at JonesL147@cardiff.ac.uk and the Clinical Research Facility email (clinical.researchfacility@wales.nhs.uk).
- By reviewing the Cardiff University Data Protection Policy: www.cardiff.ac.uk/public-information/policies-and-procedures/data-protection
- by contacting the Cardiff University Data Protection Officer by email: inforequest@cardiff.ac.uk or in writing to:
Data Protection Officer
Compliance and Risk, University Secretary’s Office
Cardiff University
McKenzie House
30-36 Newport Road
Cardiff
CF24 0DE
What happens if I choose to withdraw from the study?
You can stop being part of the study at any time, without giving a reason, this will not affect the standard of care you will receive. If you decide to withdraw from the study after providing blood samples, we would still like to use your samples/data in the study. If you wish to withdraw from the study at any time, please contact the study lead or member of the research team to discuss your options.
Are there any risks or disadvantages from taking part?
We do not foresee any significant risks to you as a result of taking part in this study. SPDLife has been well studied and there is evidence that it is a safe supplement. There are no known side effects to SPDLife. Taking any supplement may lead to some minor side effects (for example an unpleasant taste, an upset stomach or bloating) and we will monitor these. The disadvantages would be taking a few minutes of your time to consider taking part in the study, the minor discomfort having a blood sample and the inconvenience of taking supplements and making a note of any side effects during the study.
Are there any benefits from taking part?
We cannot promise the study will benefit you directly, but the information gathered through this study aims to improve global knowledge of vaccination and inform future clinical practice for prevention and treatment of infection outbreaks and improve patient care and outcomes.
What will happen to the results of this study?
At the end of the study the information collected will be analysed. You will not be identified in any report or publication. We plan to share the results through presentation at conferences and publication in journals. Your study doctor will be informed of any publications and will be able to supply a copy of these publications to you on request and newsletters. We can also inform you whether you took the placebo of the supplement and the results of the research, at the end of this study by leaving your contact details on the consent form.
What if there is a problem?
If a participant in research is considered to have suffered harm through their participation, the NHS redress scheme will be used. Cardiff University is a member of UM Association Limited for insurance purposes. If you have a concern about any aspect of this study, please speak to the relevant researcher (telephone 02921 848251) who will do their best to answer your query. If you remain unhappy and wish to complain formally, you can contact Cardiff and the Vale University Health Board’s Concerns team by telephone (02921836318).
Who is organising and funding the research?
This study is being organised by Cardiff and the Vale University health board, Cardiff University and the University of Oxford. Cardiff and the Vale University health board is conducting the clinical research and Cardiff University and the University of Oxford is performing the laboratory experiments. Cardiff University is the Sponsor of this study. Funding is provided by The Longevity Labs Limited and Health and Care Research Wales.
Who has reviewed the study?
This study has been reviewed by, and received ethics clearance through, Research Ethics Committee (Reference number 24/WM/0065 ). All research in the NHS is looked at by independent group of people, called a Research Ethics Committee, to protect your interests. This study has been reviewed and given favourable opinion by Black Country Research Ethics Committee. This study has been also been given approval to commence by Cardiff and the Vale University Health Board.
Data Protection
Cardiff University is the data controller with respect to your personal data and, as such, will determine how your personal data is used in the study.
Your personal data will only be processed for the purpose of the research outlined above and for quality assurance purposes.
Expenses
You are able to claim your travel expenses incurred while taking part in this study. We are unable to reimburse any accommodation costs you may incur. In order to process any expenses, personal information that is required for financial reimbursement will be confidentially shared with finance staff at Cardiff University.
Contact for Further Information:
Dr Lucy C Jones,
Address: Clinical Research Facility, Upper Ground Floor (C Block) University Hospital of Wales Heath Park, Cardiff CF14 4XW.
Email: JonesL147@cardiff.ac.uk and clinical.researchfacility@wales.nhs.uk.
Telephone: 02921848251 or 07480926235
Thank you for taking the time to consider taking part in the study
PIS version 3. 17.04.2024 IRAS 314310.
