World AMR Awareness Week 2023: The Centre for Trials Research I3 Division
17 November 2023
World AMR Awareness Week: The Centre for Trials Research I3 Division highlight the research they do to improve diagnosis of infections to guide appropriate antibiotic use and reduce the spread of antimicrobial resistance.
Background
Antimicrobial Resistance (AMR) is an urgent global public health threat, whereby increasing numbers of bacteria are becoming resistant to commonly used antibiotics that are designed to kill them. This resistance causes approximately 700,000 deaths each year and can affect people of all ages. It has significant impacts on healthcare, veterinary, and agricultural industries and has become a primary focus of research over recent years.
World AMR Awareness Week (WAAW), (previously World Antimicrobial Awareness Week) is a global campaign that is celebrated annually from November 18th-24th to improve awareness and understanding of AMR, and aims to encourage best practices among the public, healthcare stakeholders, and policymakers, who all play a critical role in reducing the further emergence and spread of AMR. To curtail AMR, and encourage better stewardship of antibiotic use, it is vital that antibiotics are used appropriately in clinical care, guided by diagnostic tests that differentiate between viral or bacterial infection.
The Centre for Trials Research (CTR) has extensive expertise in designing and delivering AMR related research, predominantly delivered within the Infection, Inflammation and Immunity (I3) division. Many of our studies are funded by the National Institute of Health and Care Research (NIHR) who support research on antibiotic stewardship, vaccine development and diagnostics, as well as other high priority research in diverse areas.
The I3 division currently has three distinct research themes: infectious disease, vascular disorders, and immune-mediated inflammatory conditions. To highlight work within our Infectious Diseases theme and raise awareness of AMR during WAAW, we are showcasing our portfolio of infections trials.
Infections trials and other diagnostic studies coordinated by the CTR I3 division

Many of our studies focus on biological markers (biomarkers) of infection, which are present in the blood during infection, and can be easily measured by a diagnostic test. The majority of our studies focus on one particular biomarker, Procalcitonin (PCT), as levels rise early in people with serious bacterial infections. However, there are other biomarkers of infection which can be measured, and further research will be focused on comparing effectiveness of using these biomarkers to help clinicians better diagnose bacterial infection and prescribe antibiotics in an appropriate manner. Some of our studies are summarised below:
BATCH

BATCH (Biomarker-guided duration of Antibiotic Treatment in Children Hospitalised with confirmed or suspected bacterial infection), sponsored by the University of Liverpool, is a prospective two-arm individually randomised controlled trial (RCT) of children aged between 72 hours old and up to 18 years old admitted to hospital, and being treated with intravenous (IV) antibiotics for suspected or confirmed bacterial infection.
Research Aims: To determine if the addition of PCT testing to current best practice based on the NICE Antimicrobial Stewardship (AMS) guidelines can safely allow a reduction in duration of antibiotic therapy compared to current best practice alone. (Sample size = 1942).
Progress: BATCH has finished recruiting and is in the analysis phase. Results are expected in January 2024.
PEACH
PEACH (Procalcitonin: Evaluation of Antibiotic use in COVID-19 Hospitalised Patients), sponsored by the University of Leeds, is a multi-centre retrospective, observational data study of adults admitted to hospital with COVID-19 (first wave).
Research Aims: To assess whether the use of PCT testing, to guide antibiotic prescribing, safely reduced antibiotic use among patients who were hospitalised with COVID-19 during the first wave of the pandemic. PEACH is made up of three distinct work packages with different sample sizes. The main work-package (WP 2.1) includes data sourced from ~ 7000 COVID-19 patients from 11 NHS acute hospitals.
Progress: PEACH has finished data collection, with several publications already in the public domain from two work packages. Results of the main work package are expected in December 2023.
PRECISE
PRECISE (MR-PRo-adrenomedullin (MR-proADM) and ImmunoXpert Evaluation of procalcitonin-guided antibiotic duration in Children with Infection for Stratification of Effectiveness), sponsored by the University of Liverpool, is an embedded mechanism of action study within the BATCH trial and includes the same population of children.
Research Aims: To determine if there are specific sub-groups of patients (based on host response and organ dysfunction), for whom a PCT-guided antibiotic algorithm may be beneficial, harmful or ineffective. Aims to identify endotypes or sub-phenotypes of infection to facilitate optimisation of antibiotic dosing and duration (when, by how much and for how long). Sample size = 266 baseline blood samples.
Progress: All blood samples have been collected and are being analysed. Results are expected in January 2024.
PRONTO
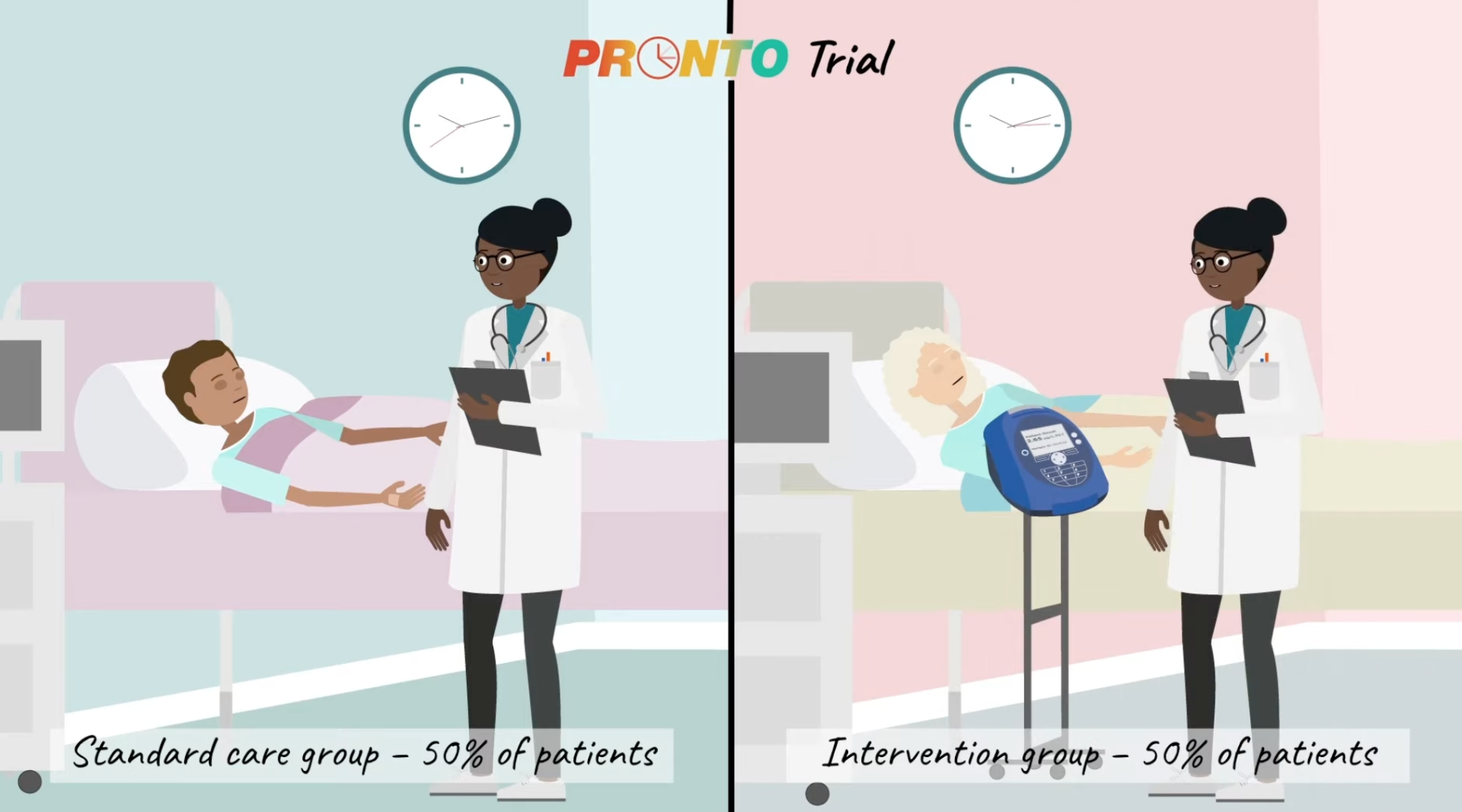
PRONTO (PROcalcitonin and NEWS2 evaluation for Timely identification of sepsis and Optimal use of antibiotics in the Emergency Department), sponsored by University of Liverpool, is a phase III, Prospective, individually randomised, open label, two arm, group sequential RCT of adults and adolescents ≥ 16 years presenting to emergency care departments with suspected sepsis. (see our video summary PRONTO video)
Research Aims: To assess whether the addition of PCT measurement to a National Early Warning Score (NEWS2) leads to a reduction in IV antibiotic initiation with no increase in 28-day mortality compared to NEWS2 scoring alone in the management of patients seen in the Emergency Department (ED) with suspected sepsis. Sample size = 7,676.
Progress: PRONTO has recently finished recruiting and is in the analysis phase. Results are expected in May 2024.
PROTECT
PROTECT (Platform Randomised evaluation of clinical Outcomes using novel diagnostic TEChnologies to optimise antimicrobial Therapy) is a NIHR Accelerator Award to carry out preparatory work for an application to a NIHR HTA programme commissioned call on platform trials.
It is a phase III, adaptive, multi-arm multi-stage (MAMS) platform trial to assess the effectiveness, implementation, and efficiency of biomarker-guided antimicrobial stewardship interventions, to reduce unnecessary antibiotic usage by excluding severe bacterial infection in acutely unwell patients.
Research Aims: To assemble a multidisciplinary team of researchers who can plan and prepare a well-designed platform trial, that allows multiple technologies to be evaluated rapidly, and then to be adopted quickly into care to benefit patients. We need to show that these technologies are safe for patients, improve care, and reduce the use of unnecessary antibiotics.
Results: We are preparing a Stage 1 submission to the NIHR HTA commissioned call.
If funding is successful, the PROTECT platform will be run across multiple UK sites. One of these sites is anticipated to be the University Hospital of Wales (UHW). Dr Jordan Evans is a paediatric consultant in emergency medicine and will be leading the research at the Children’s Hospital at UHW.
“As a consultant in Paediatric Emergency Medicine, I frequently encounter challenging clinical scenarios involving the risk stratification of unwell febrile children. Striking a balance between practising effective antimicrobial stewardship and ensuring that I don’t overlook the ‘needle in the haystack’ that is sepsis. I will be thrilled to assume the role of Co-Investigator and Principal Investigator at the University Hospital of Wales for the PROTECT platform study. This important study aims to identify innovative and precise diagnostic tests that will empower both myself and my colleagues to make safe and timely clinical decisions”
Jordan Evans, PEM Consultant, UHW, and Treialon Cymru Associate at CTR.
Jordan is also an associate member of Treialon Cymru, an exciting initiative funded by Health and Care Research Wales (HCRW) to provide opportunities across the whole of Wales for people to engage with trials. It is led by the Centre for Trials Research at Cardiff University and links across the Health and Care Research Wales funded infrastructure and beyond.
Sore Throat Test and Treat (STTT) service
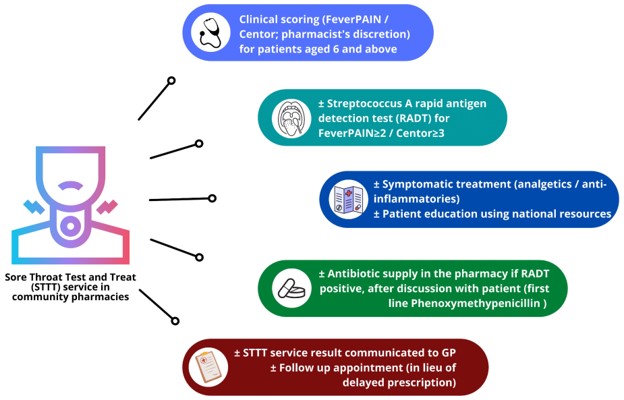
An NHS-funded sore throat test and treat (STTT) service was introduced in selected community pharmacies in Wales. Service users are screened using FeverPAIN/Centor scores, offered rapid antigen detection testing to detect group A Streptococcus if appropriate, and supplied with antibiotics (by the pharmacist) if indicated. Following an initial pilot, the service was rolled out nationally.
Research Aims: A collaboration between the School of Pharmacy, Division of Population Medicine, and CTR has led to a long-term evaluation of the STTT service. This was accomplished by (a) describing the service to date over a 16 months period; (b) comparing data for the pre-pandemic to the pandemic period when routine POCT was removed; (c) comparing STTT service users to sore throat GP consultations for antibiotic supply, reconsultations, and complications. To achieve our aims, individual-level STTT consultation data were extracted from electronic pharmacy records, and linked to SAIL Databank.
Results: Over 16 months, 11,304 STTT consultations were completed with a 21% antibiotic supply rate, significantly lower than prescribing rates from GP sore throat consultations. Overall antibiotic supply rate increased by 27% when routine POCT was removed. The work to date concludes that STTT continues to offer a safe option for patients; it can be delivered at scale to align with a pre-specified pathway that promotes appropriate use of POCT and antibiotics. This has significant implications for antimicrobial stewardship and is the rationale behind the re-introduction for the requirement for routine POCT.
Progress: The results from the data linkage work will be reported in early 2024.
The I3 division has strong links with several external collaborators and below are the Chief Investigators we are currently working with on the studies highlighted above.
- Professor Enitan Carrol, Professor of Paediatric Infection
- Professor Neil French, Professor of infectious diseases and global health
- Dr Stacy Todd, Infectious diseases consultant at Royal Liverpool University Hospital
- Dr Jonathan Sandoe, Associate clinical professor of microbiology.
- Dr Efi Mantzourani, Reader in Cardiff University, School of Pharmacy
Research Impact and future direction on infections trials
“The use of rapid diagnostics is increasingly recognised as an important tool for ensuring the prudent use of antimicrobials. Within the CTR, the evaluations carried out are addressing vital clinical questions with practice-changing implications. Trials of antimicrobial stewardship interventions require complex trade-offs, and within the CTR we have pioneered the use of co-primary outcomes in this field and have extended this to include embedded interim analysis. All of this ensures we are reliably answering questions of importance in an efficient manner. Our next step will be to use our collective expertise and extensive networks to establish a platform infrastructure which enables multiple interventions to be evaluated in perpetuity. This approach has been found to be successful in other clinical areas and is the natural next step for the field of antimicrobial stewardship if changes in practice are to keep pace with the growing consequences of AMR.”
– David Gillespie, Director of Infection, Inflammation, and Immunity Trials, Centre for Trials Research
Authors: Jo Euden, Cherry-Ann Waldron, Waku Maboshe, Emma Thomas-Jones, Rebecca Cannings-John, David Gillespie, on behalf of all staff involved in the name studies above.
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016