Raising Awareness Of PAD, Pain And Vascular Disease: The PLACEMENT Trial
25 September 2023
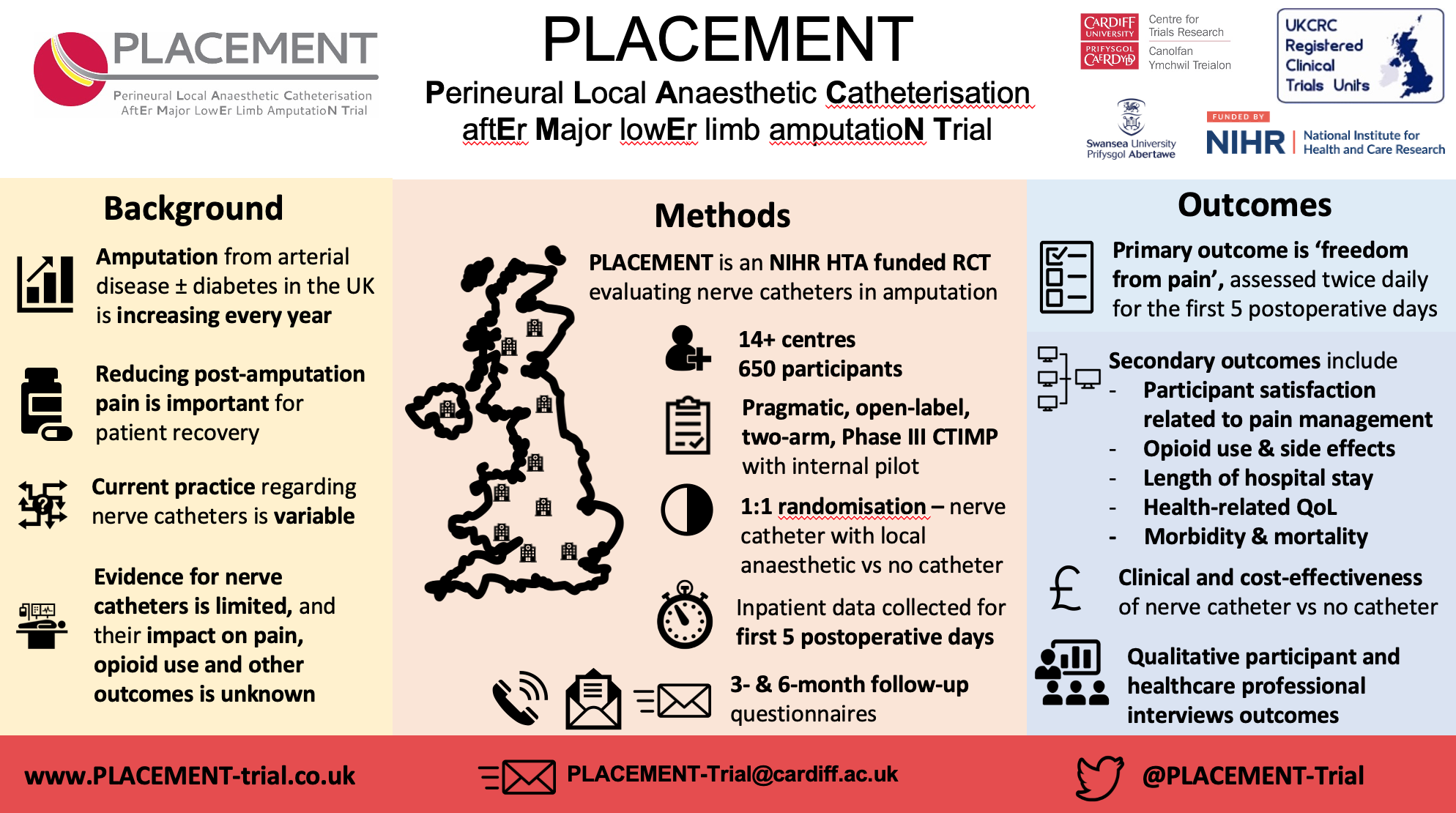
This month is peripheral arterial disease (PAD) awareness month, vascular disease awareness month and pain awareness month. The number of adults with age-related chronic diseases, including PAD/vascular disease, is increasing worldwide. Similarly, the number of adults who undergo leg amputation surgery for PAD/vascular disease in the UK is increasing each year. Pain experienced after leg amputation surgery is a leading cause of patient dissatisfaction and a barrier to their rehabilitation, which may affect their recovery. The management of patient’s pain is therefore important for them to achieve their short- and long-term goals after amputation surgery. We held a discussion group with amputees who helped us design this trial by telling us what is important to them about amputation pain. The Perineural Local Anaesthetic Catheter After Major Lower Limb Amputation Trial (PLACEMENT) has been designed to compare postoperative pain in two groups of people undergoing leg amputation surgery for PAD/vascular disease.
What is planned?
650 patients with planned leg amputations for PAD/vascular disease will be recruited from 14 UK NHS sites, including eight lead sites. Cardiff and Vale University Health Board is the lead site, with their Principal Investigator, David Bosanquet, also the Chief Investigator of the trial. The seven other lead sites include Bristol, Swansea, Imperial, Leicester, Newcastle, Hull and Cambridge. Cardiff and Vale and Leicester have had their site initiation visits in the last two weeks, and we hope to initiate the remaining six lead sites over the next four weeks.
At the time of their leg amputation surgery, participants will be randomised to either receive a perineural nerve catheter (PNC) or not. A PNC is a thin plastic tube, placed next to the main leg nerve which is cut during amputation surgery. Participants randomised to receive the PNC will have a local anaesthetic infused for the first five days after leg amputation surgery. All participants will receive additional pain-management medications as required. The primary aim of PLACEMENT is to evaluate if the use of a PNC with continuous local anaesthetic infusion, affects the amount of pain participants experience, compared to no PNC. Secondary aims include assessing the effect of PNC use on participant satisfaction of pain management, and recovery at 3 and 6 months, including chronic pain, wound healing, quality of life, time to achieve prosthesis fitting and level of independence.
Recommendations for researchers
The findings from this research will be presented at medical conferences, published in free to access medical journals, and shared with people who write amputation surgery guidelines and policies. We will share the trial results via the national press, amputation charities and social media, including the PLACEMENT Twitter and website pages. Our patient and public involvement (PPI) group will work with us throughout the trial and help prepare a summary of the results. Two members of the public are a central part of our research team. One has had two amputations, one with and one without a PNC. Our other member lost her father after an amputation. Both helped us plan and run the PLACEMENT Feasibility trial and plan this larger trial.
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016