PATHOS Clinical Trial: Exploring De-Intensification in Head and Neck Cancer
27 July 2024The PATHOS clinical trial is investigating the potential benefits of de-intensified treatment for individuals with head and neck cancer, specifically HPV-positive oropharyngeal cancer. Led by Professors Terry Jones and Mererid Evans, the trial is funded by Cancer Research UK, co-sponsored by Cardiff University and Velindre University NHS Trust and managed by the Centre for Trials Research at Cardiff University.
The recruitment target of 1,269 participants has been achieved, and we acknowledge the fantastic support from participants and sites. The study aims to compare the outcomes of less intensive treatment against standard treatment. The primary focus is on whether a reduced intensity approach can minimize swallowing difficulties without compromising the effectiveness of the treatment.
All participants undergo surgery to remove their cancer, a procedure that remains consistent regardless of trial participation. Following surgery, a pathologist examines the cancer tissue under a microscope and identifies key features, such as the cancer stage, which help doctors determine the necessity and type of additional treatment.
Participants in Group A do not require further treatment, but still have follow up visits with the trial team.
Participants in Group B will have radiotherapy.
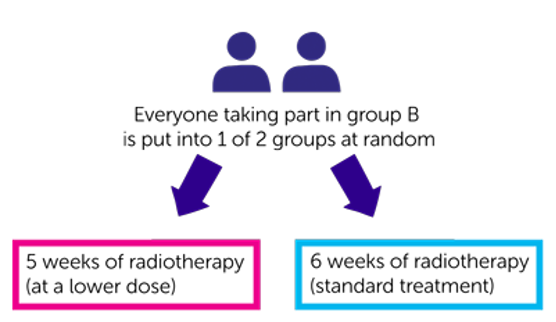
Participants in Group C will have radiotherapy alone or radiotherapy and chemotherapy.

The PATHOS trial represents a significant step towards optimizing cancer treatment by potentially reducing side effects while maintaining efficacy.
Feedback from PATHOS Participants
A Cardiff dad-of-two who thanks research for saving his life has been chosen to launch STAND UP TO CANCER in Wales. Read more at https://www.walesonline.co.uk/news/health/painless-lump-mentioned-way-out-28427376
A throat cancer survivor says a pioneering clinical trial has “helped me get back to enjoying life with my wife”.
Another participant reported Taking part in the trial has “given me a lot more mental security” as he has been in hospital and “I’m constantly checked up on it”.
Read more at https://www.bbc.co.uk/news/articles/c3g9j48n649o
Another participant wrote to the study team with the following feedback:
After six years of following a lower dosage of radiotherapy, I am grateful to report that I can swallow normally, except for rare difficulties with very sticky foods, thanks to the dedication of a multidisciplinary team of health professionals. As an engineer, I found that seeing x-ray footage of my swallowing during the PATHOS study greatly enhanced my understanding of the importance of swallowing exercises, and I suggest that similar visual aids might help other patients comprehend and adhere to their exercises.
The trial closes recruitment in October 2024, with results expected early 2028. The study team is so grateful to all the sites and all the wonderful participants who have supported this trial.
- December 2025
- October 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- December 2024
- November 2024
- October 2024
- September 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016