One Year Of The PRONTO Trial: Challenges Observed And Overcome During A Global Pandemic
18 November 2021
What Is Sepsis?
Sepsis, blood poisoning, septicaemia…whatever word you are familiar with, it remains one of the most common complications of infection seen in hospital Emergency Departments (ED)s across the UK and is characterised by an unregulated host response to bacterial infection. Optimal treatment for sepsis includes prompt antibiotics into a vein (intravenous/IV) and currently, clinicians assess severity of illness in patients in the ED with a scoring system based on simple to measure observations: the National Early Warning Score (NEWS2).
Adults with suspected sepsis fall into one of three categories: a) those looking ill and need urgent IV antibiotics and fluids within 1 hour, b) those that are unwell, but will not come to harm if IV antibiotics are not administered within 1 hour, allowing time for further assessment prior to starting antibiotics and c) those not critically unwell who may or may not need IV antibiotics.
Why Research Is Needed Now
The identification, assessment and management of sepsis is challenging because of its many non-specific symptoms and signs, caused by both infectious and non-infectious diseases. The NEWS2 scoring system helps clinicians identify the sickest patients but it is not specific, and tends to over-diagnose sepsis with up to 50 % of patients who are initially managed as sepsis, not receiving a final diagnosis of such. In line with international recommendations, National Institute for Health and Care Excellence (NICE) sepsis guidelines suggest the administration of intravenous antibiotics within an hour to patients who are considered to be high risk for admission to Intensive Care Units (ICU) and death. This leads to over-prescribing of antibiotics which promotes antimicrobial resistance (AMR), a global problem! It is the best we have and currently used in over 70% of English hospitals.
PRONTO
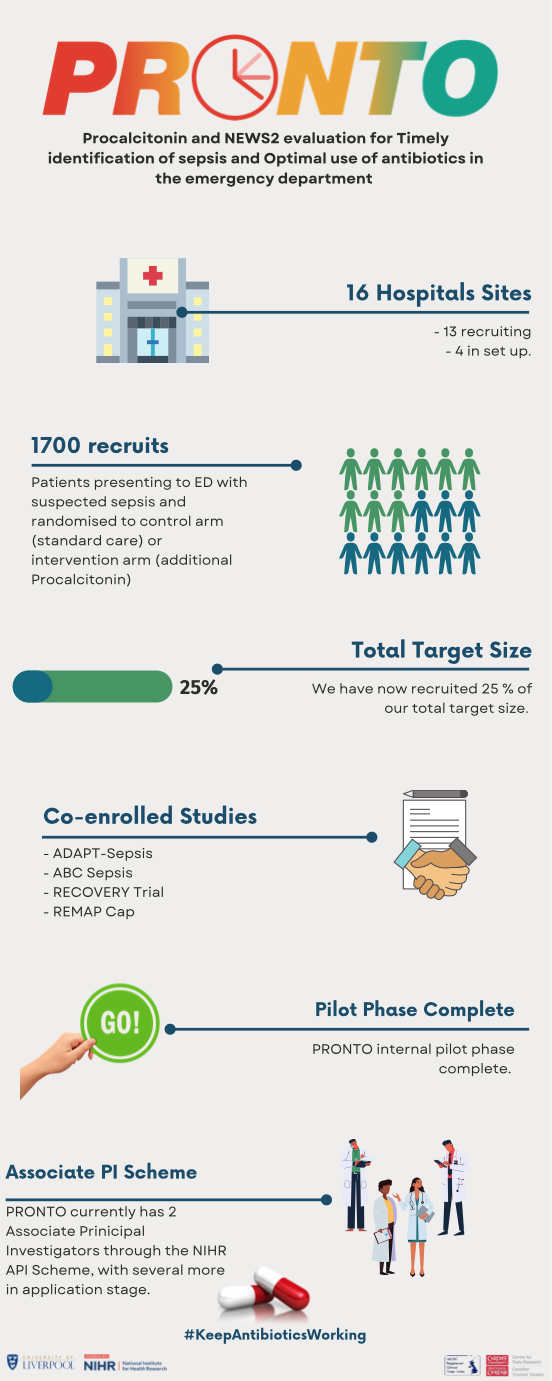
Procalcitonin (PCT) is a reliable biological marker present in the blood that changes early in the course of bacterial infection. It is not widely used across the NHS currently but has been evidenced to identify bacterial infections and to better inform decisions on when to prescribe antibiotics. The PRONTO Trial (Procalcitonin and NEWS2 evaluation for Timely identification of sepsis and Optimal use of antibiotics in the emergency department) is answering a call from NICE that recommends further research on PCT testing in EDs for guiding antibiotic use in people with suspected sepsis.
What Does The PRONTO Trial Involve?
PRONTO is an NIHR-HTA funded randomised controlled trial (RCT), sponsored by the University of Liverpool, that compares PCT-supported assessment with standard care of suspected sepsis in adults presenting to the ED, and measures whether this approach reduces prescriptions of antibiotics without increasing mortality by decreasing uncertainty in the group who may not need IV antibiotics urgently within 1 hour, or not need antibiotics at all. In the PCT arm, a bedside test (taking 20 minutes), known as a Point of Care test (POCT) is performed plus the NEWS2 assessment. Depending on the result of the PCT plus the NEWS2, patients receive IV antibiotics and fluids within the current recommended time frame depending on severity. Doctors and patients know what treatment arm they are in and doctors remain free to use antibiotics outside the study guidelines using their clinical judgement in any of the risk groups.
The objective of PRONTO is not to prevent patients who require antibiotics from receiving them, but to assess whether by using NEWS2 and PCT, we can identify patients who require antibiotics within 1 hour whilst providing more time for clinicians to decide if antibiotics are required for patients who are less severely ill. It may therefore lead to less antibiotics and more narrow spectrum antibiotics being prescribed as clinicians may have more time to assess patients before they prescribe antibiotics. The rapid result of the PCT test, especially if it indicates a low or medium risk, may also provide reassurance to patients and families, and reduce anxiety.
Challenges Faced So Far
When we first started setting up PRONTO, we aimed to have 10-14 sites taking part in the trial, collectively recruiting 7676 adult patients with suspected sepsis to be randomised to current standard care or PCT-supported care. As with many clinical trials, we had a sponsor and NIHR imposed delay to opening, finally opening our first site (Royal Derby Hospital) in November 2020.
Clinical trials conducted within ED/critical care settings are challenging as the need to administer potentially life-saving treatments in a timely manner, patient safety and adherence to NHS reporting guidelines come first and foremost above research. Due to the need for rapid assessment and treatment of patients with suspected sepsis, PRONTO uses a deferred consent model as giving enough time to patients to make a fully informed decision on whether to give consent cannot be done in such tight time frames. When our first sites were opening, problems obtaining informed consent and the ethical issues surrounding the procedures in place presented the greatest obstacle for NHS staff. As is still the situation now, relatives and loved ones are unable to visit patients in hospital. We have spent considerable time adjusting existing documents and implementing additional procedures in order to make the consent process both more effective and efficient for our sites.
Milestones And Successful Events
It has not all been delays and adjustments. To align with COVID research recommendations and to make PRONTO more relevant to the current situation in EDs, we incorporated changes into our protocol that allowed us to collect data on COVID diagnosis so we could build this into our analysis. This meant we were successful in registering PRONTO as an Urgent Public Health (UPH) study whilst we were at the height of the pandemic. What this meant is that NHS Trusts were obliged to keep research going that was badged as a UPH Trial. It’s an incredible achievement for all of the staff involved in PRONTO to recruit participants into research in such a busy and demanding environment and in such difficult times. As we enter into another winter season where seasonal infection rates start to soar, we do not know what fresh new challenges we face but recruitment remains steady and continuous.
We also registered PRONTO for the NIHR Associate Principal Investigator (API) scheme in March this year. The aim of the API scheme is to develop the Principal Investigators (PIs) of the future and it is open to junior doctors, nurses and allied health professionals whose current role does not contain a significant research component. On taking on the Associate PI role, an individual commits to a minimum of 6 months supporting the local delivery and organisation of the PRONTO study, with recognition of the work undertaken in the role endorsed by NIHR certification at the end of tenure, recognition in PRONTO publications, professional development and experience to take forward as a future PI. Currently, PRONTO has two registered APIs. With these coming to the end of the tenure, we are currently in the process of junior doctor turnovers across our sites and identification of new API registrants.
November 18th 2021 marks the first complete year of recruitment into PRONTO with Royal Derby Hospital recruiting their first participant on the first day of opening. We currently have 13 recruiting NHS hospitals with a further 3 identified and moving through the set-up process. At the time writing this, PRONTO has just come to the end of the pilot phase, where we assess feasibility and acceptability of the trial at our sites. We have recruited 1679 patients from our total target sample size of 7676 – a veritable achievement!
Patient and Public Involvement (PPI) And Dissemination Of Public Information
To ensure that all patient facing materials in a trial are presented in a suitable way, PPI representatives are imperative to any good trial design. We have a very experienced PPI representative in PRONTO whose experience has been, and continues to be, invaluable throughout the life-cycle of the project. As a sepsis survivor herself, JC has been able to give insight into patient perspectives when considering trial design and has contributed to the logistics of the day to day running of the trial. PRONTO also benefitted at the design stage from several PPI advisory groups, several of whom have had sepsis, or had a relative with sepsis.
As we use a deferred consent process, the information that we present to the public, and the way in which this is done, is key. The input of PPI members to guide design of information leaflets and posters in PRONTO adds an element of communication that is often forgotten amongst trial study teams. To further aid public communication, we commissioned a short 1.5 minute animation, outlining the main aims of the PRONTO trial. A social media campaign and corresponding impact report by the design company showed that engagement was high and reached over 60,000 users with engagement from around 1,600 social media users. The take home message here is never underestimate the power of public engagement and social media! If you want to find out more about PRONTO, you can watch our video here:
To summarise, it has been a great first year of PRONTO, challenges and all, and as we move from our pilot phase into our main phase of the study, I encourage you all to read up on the challenges facing us all as antimicrobial resistance continues to be one of the top 10 global threats to health facing humanity. #KeepAntibioticsWorking.
– Blog by Dr Joanne Euden
To read more about PRONTO, please visit https://www.cardiff.ac.uk/centre-for-trials-research/research/studies-and-trials/view/pronto
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016