Addressing The Knowledge Gap – Developing CONSULT E-learning On Trials Involving Adults Lacking Capacity To Consent
9 December 2024
Historically, trials have focused on the number of participants who take part without paying too much attention to whowas being included – or rather, being excluded. More recently, however, there has been growing attention on ensuring that trials are inclusive of groups who are under-served by research. This has led to funders such as the National Institute of Health and Care Research (NIHR) now making inclusion a condition of funding.
Under-served groups include those who experience lower inclusion in research than would be expected from population estimates, or where there are important differences in how a group responds to or engages with healthcare interventions compared to other groups. Both the NIHR, and more recently the World Health Organisation, have recognised that one such under-served group are people who lack the capacity to give consent for themselves.
Exclusion of people with impaired capacity to consent
People who are unable to provide their own consent are frequently excluded from trials where they are an important part of the clinical population who could benefit from the intervention. This exclusion is due to the particular ethical and legal considerations in studies including adults lacking capacity. With different legal frameworks applying in different parts of the UK and for different types of research, many researchers find trials involving adults with impaired capacity challenging to conduct.
“It feels like an insurmountable black box of horrendousness that I dare not go. It feels very much that if you get this wrong you will be illegal ….. and the ethics police will come for you … so we just try to avoid it” (Researcher, CONSULT-ENABLE Study)
In the CONSULT project which is funded by Health and Care Research Wales and explores the ethical, legal, and methodological challenges encountered in research involving adults with impaired capacity to consent, researchers told us that they would benefit from having better support. That led to us developing the INCLUDE Impaired Capacity to Consent Framework as a tool for researchers to design and conduct trials involving people with impaired capacity to consent, and a website of information and resources. Researchers also told us that they do not always have the knowledge and skills they feel they need and that they would benefit from better training in this area.
“We need better support and guidance, particularly around the correct terminology, who needs to give consent, and how it varies between different countries, to give people more confidence to set up this sort of study” (Researcher, CONSULT-ENABLE Study)
Developing the CONSULT e-learning for researchers
Funded by a UKRI Impact Accelerator Award, the training was developed over four stages, including an initial survey to explore researchers views about the current gaps in training, the main challenges they encountered when designing and conducting these trials in a range of populations and settings, and what they found worked well. It also explored their experiences of public involvement to support these trials. The project was supported by a Lay Advisory Group and a Researcher Advisory Group who helped shape the format of the e-learning and the content which was based on the findings from the CONSULT project.
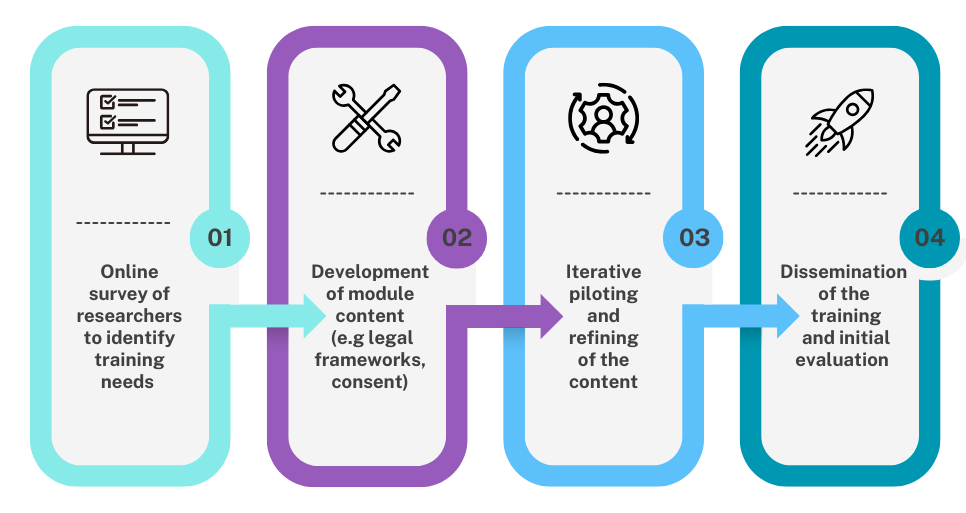
The training consists of four e-learning modules covering an introduction to the topic, the legal and ethical frameworks that apply, a deeper dive into issues around consent and consultation with a focus on supporting participants’ decision-making, and the methodological issues to consider. Each module contains videos and case studies to illustrate key content and the role of public involvement, with links to more resources throughout and collated at the end.

It is free to access via Moodle registration, and can be completed at the learner’s own pace, taking about half a day in total. On completion of the e-learning there is the option to download a certificate. It is designed to support researchers, alongside using the INCLUDE Impaired Capacity to Consent Framework, to design and conduct trials that are more inclusive of adults with impaired capacity to consent.
Launching the CONSULT e-learning
We launched the training in December at a webinar with 160 registered attendees including researchers, members of the public, research ethics committee members and others. Prof Kerry Hood, Director of UK CRC CTU Network and Dean of Research & Innovation for Biomedical and Life Sciences at Cardiff University, shared reflections on the importance of inclusivity in trials. Vicky Shepherd, who led the project with Martina Svobodova and Nicky Ivins, presented on the background to the project and how it can help researchers and Sian Jones, a member of the Lay Advisory Group, shared her personal reflections. An expert panel then provided insightful responses to questions raised by webinar attendees who shared some of the key challenges they encounter. A recording of the webinar is available, and feedback has shown that both the webinar and the training have made a valuable contribution towards shinning some light into the ‘black box of horrendousness’.
The CONSULT e-learning can be accessed via the CONSULT website, alongside instructions and a link to the launch webinar: https://www.capacityconsentresearch.com/training
We would like to thank the Lay Advisory Group, Researcher Advisory Group, and all those who kindly completed the survey.
- December 2025
- October 2025
- June 2025
- May 2025
- April 2025
- March 2025
- February 2025
- December 2024
- November 2024
- October 2024
- September 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016