Postdoctoral Research Associates

Dr Tahir Maqbool (HEC Funded PDRA, Feb 2023 – Dec 2023)
Next position: Assistant Professor, GC University Faisalabad, Pakistan
Group spatula: [link]
Publications:

Dr Shyam Basak (EPSRC Funded PDRA Oct 2019 – Nov 2020, Collaborative project with Dr Duncan Browne and Prof. Thomas Wirth; Leverhulme Trust Funded PDRA Sept 2018 – Sept 2019, Collaborative project with Dr Rebecca Melen)
Next position: Senior Scientist, Sygnature Discovery, Nottingham, UK
Group spatula: [link]
Publications:
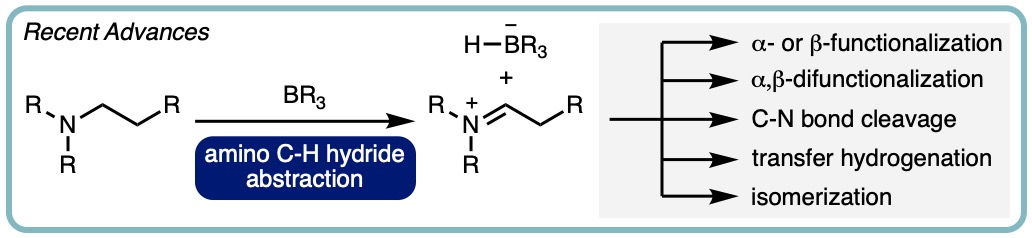
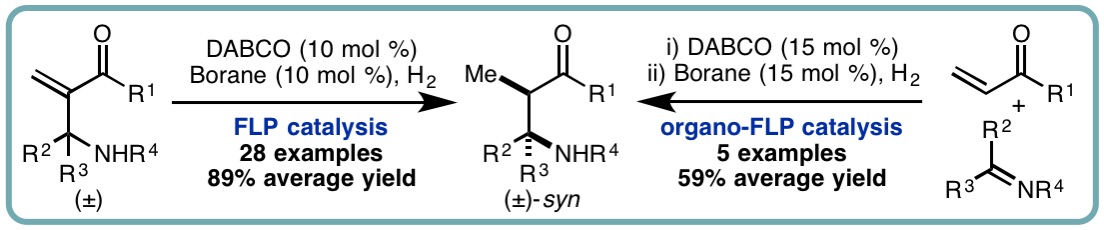
- “Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes”, S. Basak, L. Winfrey, B. A. Kustiana, R. L. Melen*, L. C. Morrill* and A. P. Pulis*, Chem. Soc. Rev., 2021, 50, 3720-3737. [link]
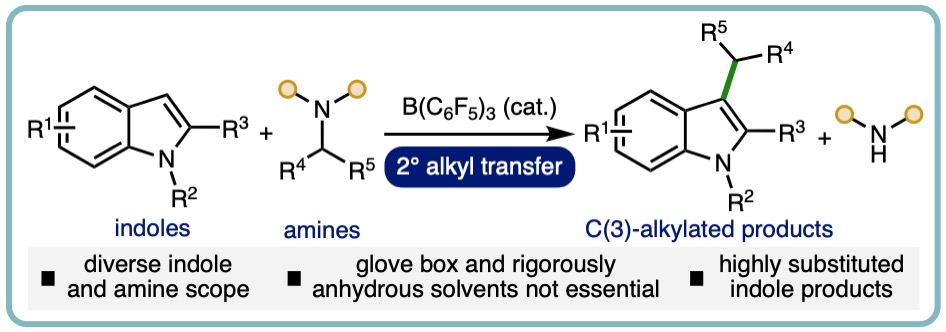
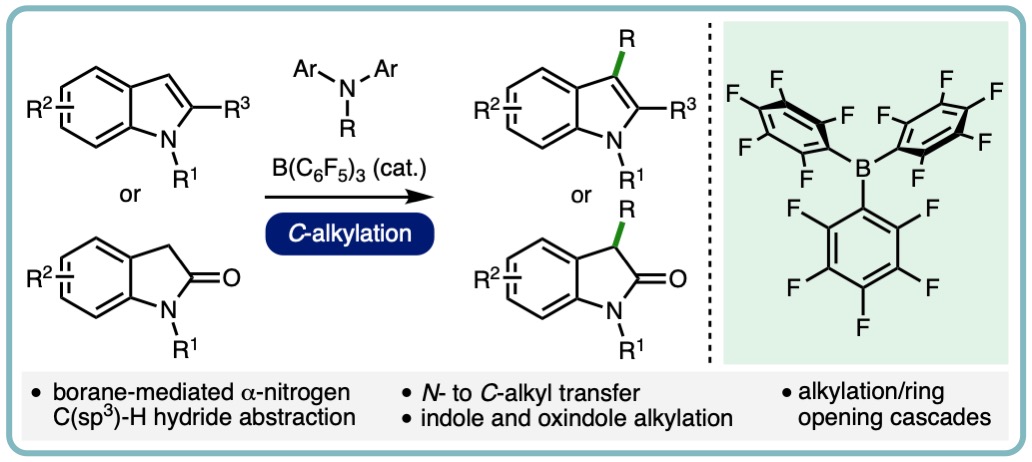
- “B(C6F5)3-Catalyzed Direct C3 Alkylation of Indoles and Oxindoles”, S. Basak, A. Alvarez-Montoya, L. Winfrey, R. L. Melen*, L. C. Morrill* and A. P. Pulis*, ACS Catal., 2020, 10, 4835-4840. [link]

Dr Ben Allen (EPSRC Funded PDRA, Collaborative project with Dr Duncan Browne and Prof. Thomas Wirth, Nov 2017 – Nov 2019)
Next position: Senior Scientist, Pharmaron, Cardiff, UK
Group spatula: [link]
Publications:
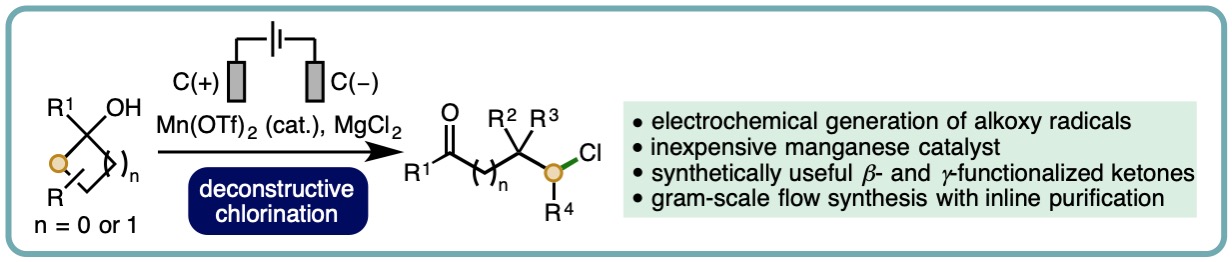
- “Manganese-Catalyzed Electrochemical Deconstructive Chlorination of Cycloalkanols via Alkoxy Radicals”, B. D. W. Allen,† M. D. Hareram,† A. C. Seastram, T. McBride, T. Wirth, D. L. Browne* and L. C. Morrill*, Org. Lett., 2019, 21, 9241-9246. [link] [Associated correction to citations] [link]
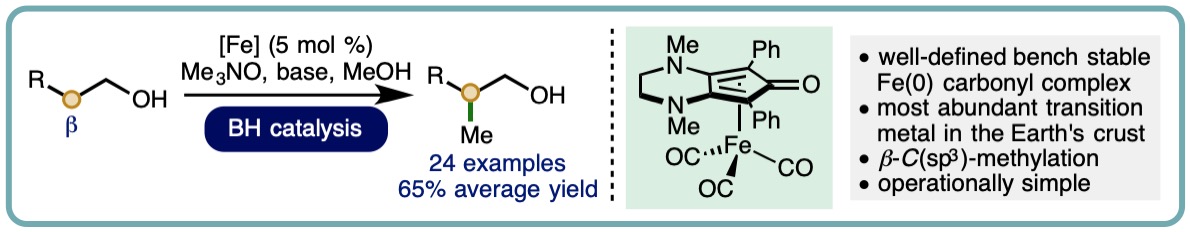
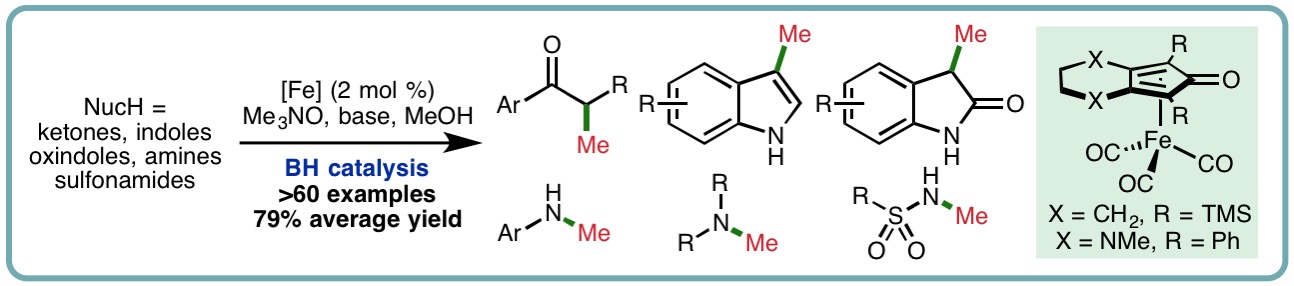
- “Iron-Catalyzed Methylation using the Borrowing Hydrogen Approach”, K. Polidano, B. D. W. Allen, J. M. J. Williams and L. C. Morrill*, ACS Catal., 2018, 8, 6440-6445. [link]
Conference Contributions:
- Enabling Technologies for Synthesis Workshop, Cardiff University, 28/06/19, Presentation

Dr Imtiaz Khan (Leverhulme Trust Funded PDRA, Collaborative project with Dr Rebecca Melen, Jun 2016 – Jun 2018)
Next position: PDRA, The University of Manchester, Advisor: Prof. James Micklefield
Group spatula: [link]
Publications:
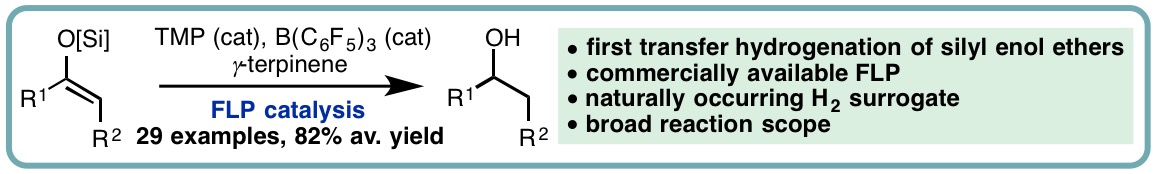
- “FLP-Catalyzed Transfer Hydrogenation of Silyl Enol Ethers”, I. Khan, B. G. Reed-Berendt, R. L. Melen* and L. C. Morrill*, Angew. Chem. Int. Ed., 2018, 57, 12356-12359. [link]
- “Frustrated Lewis Pair (FLP)-Catalyzed Hydrogenation of Aza-Morita-Baylis-Hillman Adducts and Sequential Organo-FLP Catalysis”, I. Khan, M. Manzotti, G. J. Tizzard, S. J. Coles, R. L. Melen* and L. C. Morrill*, ACS Catal., 2017, 7, 7748-7752. [link]
Conference Contributions:
- RSC Organic Division South-West Regional Meeting, University of Bristol, 17/01/18, Poster
- 254th ACS National Meeting & Exposition, Washington, DC, 20/08/17, Presentation
- 10th Molecular Synthesis Section Seminar, Cardiff University, 13/07/17, Presentation
Co-Supervised Postdoctoral Research Associates

Dr Alex Nödling (Leverhulme Trust Funded PDRA July 2017-July 2020, Collaborative project led by Dr Louis Luk)
Next position: Senior Scientist, Agenus, Cambridge Science Park, UK
Publications:
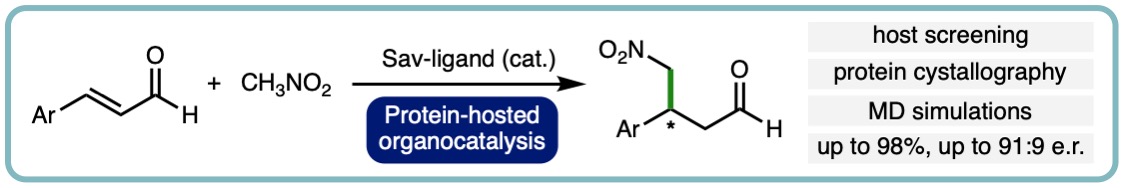
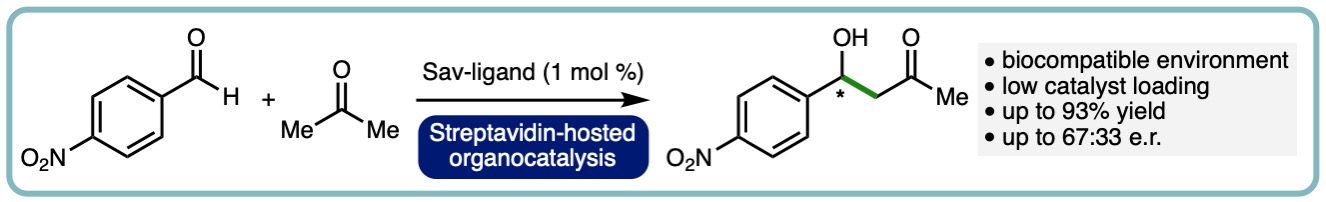
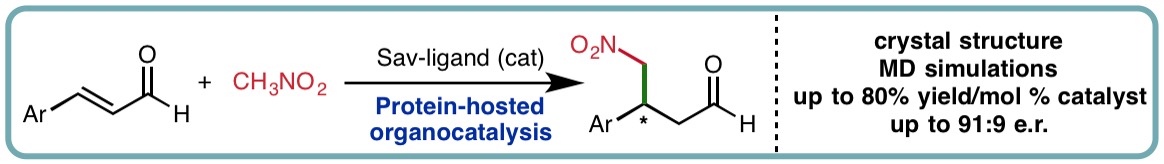
- “The Role of Streptavidin and its Variants in Catalysis by Biotinylated Secondary Amines”, A. R. Nödling, N. Santi, R. Castillo, M. Lipka-Lloyd, Y. Jin, L. C. Morrill, K. Swiderek*, V. Moliner* and L. Y. P. Luk*, Org. Biomol. Chem., 2021, 19, 10424-10431. [link]
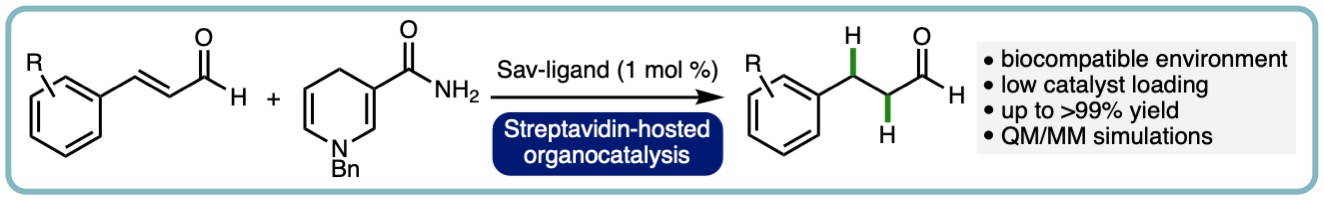
- “Reactivity and Selectivity of Iminium Organocatalysis Improved by a Protein Host”, A. R. Nödling, K. Swiderek, R. Castillo, J. W. Hall, A. Angelastro, L. C. Morrill, Y. Jin, Y-H. Tsai, V. Moliner* and L. Y. P. Luk*, Angew. Chem. Int. Ed., 2018, 57, 12478-12482. [link]
PhD Students

Albara Elgehani (AstraZeneca CASE Award PhD student, Oct 2019 – Jan 2024)
Ph.D. Examiners: Prof. Richard Brown (external), Dr Heulyn Jones (internal)
Next position: TBC
Group spatula: [link]
Publications:
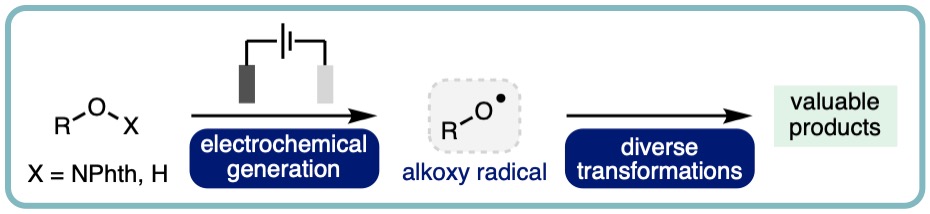
- “Electrochemical Generation and Utilization of Alkoxy Radicals”, Albara, A. M. A. El Gehani, Hussain A. Maashi, James Harnedy and Louis C. Morrill*, Chem. Commun., 2023, 59, 3655-3664. [link] [Part of themed collection: Chemical Communications Hot Articles 2023]
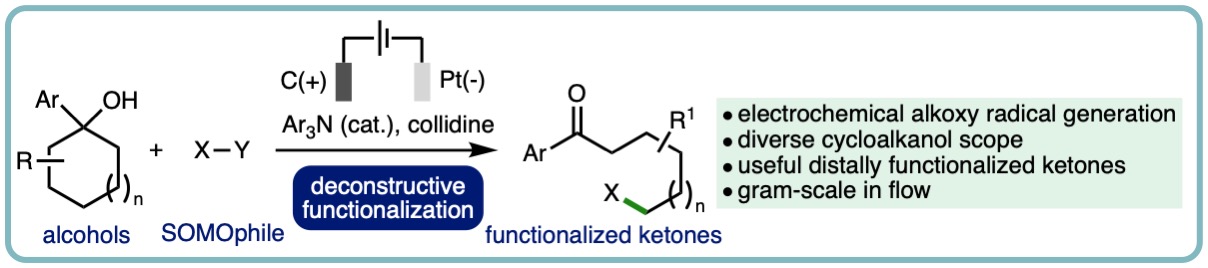
- “Deconstructive Functionalization of Unstrained Cycloalkanols via Electrochemically-Generated Aromatic Radical Cations”, James Harnedy, Hussain A. Maashi, Albara, A. M. A. El Gehani, Matthew Burns and Louis C. Morrill*, Org. Lett., 2023, 25, 1486-1490. [link]
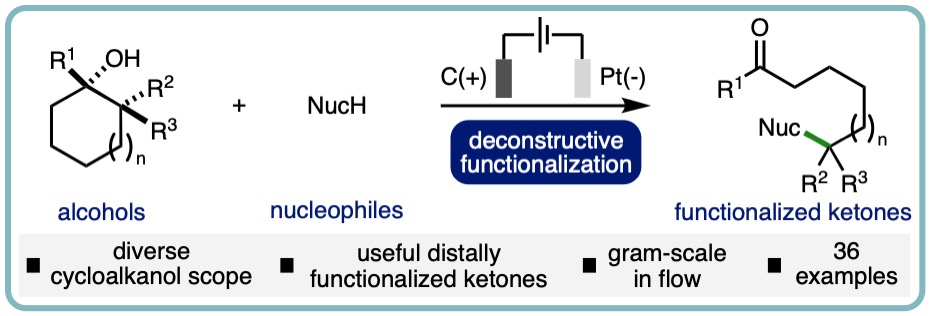
- “Electrochemical Deconstructive Functionalization of Cycloalkanols via Alkoxy Radicals Enabled by Proton-Coupled Electron Transfer”, Mishra Deepak Hareram, Albara A. M. A. El Gehani, James Harnedy, Alex C. Seastram, Andrew C. Jones, Matthew Burns, Thomas Wirth, Duncan L. Browne and L. C. Morrill*, Org. Lett, 2022, 24, 3890-3895. [link]
Conference Contributions:
- GSK Synthetic Organic Chemistry Postgraduate Symposium, 06/10/2022, Presentation
- Charles River Poster Competition, 23/09/2022, Poster
- AZ Macclesfield iCASE Symposium, 12/09/2022, Presentation
- Sygnature Discovery Postgraduate Symposium, 19/07/2022, Presentation
- 20th Cardiff Chemistry Conference, 20/05/2022, Poster
- 32nd SCI FCG Postgraduate Symposium, 13/05/2022, Presentation
- Bristol Electrosynthesis Meeting, Online, 04/04/2022, Poster [1st Place Prize]
- CatSci & LabLinks Poster Competition, Cardiff, 15/02/2022, Poster
- 34th Molecular Synthesis Section Seminar, Cardiff University, 15/07/21, Presentation

James Harnedy (PhD student Oct 2020 – Nov 2023)
Ph.D. Examiners: Dr Jon Wilden (external), Prof. Thomas Wirth (internal)
Next position: Scientist, Domainex, Cambridge, UK.
Group spatula: [link]
Publications:
- “Electrochemical Generation and Utilization of Alkoxy Radicals”, Albara, A. M. A. El Gehani, Hussain A. Maashi, James Harnedy and Louis C. Morrill*, Chem. Commun., 2023, 59, 3655-3664. [link] [Part of themed collection: Chemical Communications Hot Articles 2023]
- “Deconstructive Functionalization of Unstrained Cycloalkanols via Electrochemically-Generated Aromatic Radical Cations”, James Harnedy, Hussain A. Maashi, Albara, A. M. A. El Gehani, Matthew Burns and Louis C. Morrill*, Org. Lett., 2023, 25, 1486-1490. [link]
- “Electrochemical Deconstructive Functionalization of Cycloalkanols via Alkoxy Radicals Enabled by Proton-Coupled Electron Transfer”, Mishra Deepak Hareram, Albara A. M. A. El Gehani, James Harnedy, Alex C. Seastram, Andrew C. Jones, Matthew Burns, Thomas Wirth, Duncan L. Browne and L. C. Morrill*, Org. Lett, 2022, 24, 3890-3895. [link]
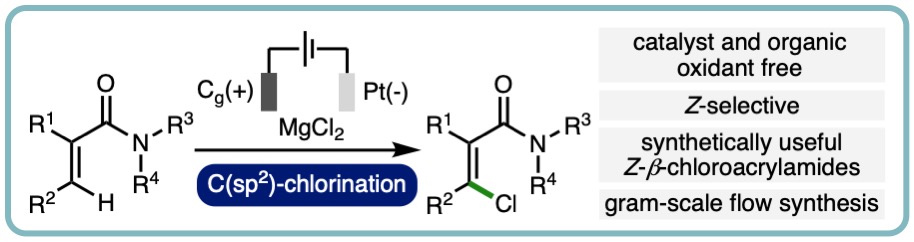
- “Electrochemical oxidative Z-selective C(sp2)-H chlorination of acrylamides”, J. Harnedy, M. D. Hareram, G. J. Tizzard, S. J. Coles and L. C. Morrill*, Chem. Commun., 2021, 57, 12643-12646. [link] [Invited contribution to the 2021 Emerging Investigators themed collection]
Conference Contributions:
- 21st Cardiff Chemistry Conference, 16/05/2023, Presentation
- 37th Molecular Synthesis Section Seminar, Cardiff University, 17/03/22, Presentation

Betty Kustiana (Faculty for the Future Scholarship and Vice-Chancellor’s Scholarship for Research Excellence PhD student, Oct 2019 – Mar 2023)
Ph.D. Examiners: Dr Ben Partridge (external), Prof. Ian Fallis (internal)
Next Position: Scientist, Selvita, Kraków, Poland.
Group spatula: [link]
Publications:
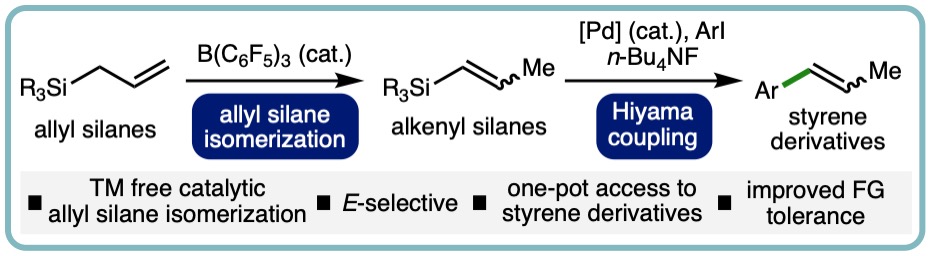
- “One-Pot Synthesis of Styrene Derivaties from Allyl Silanes via B(C6F5)3-Catalyzed Isomerization – Hiyama Coupling”, Betty A. Kustiana, Rebecca L. Melen, and Louis C. Morrill*, Org. Lett.,2022, 24, 8694-8697 . [link] [Highlighted in Chemistry Views] [link]
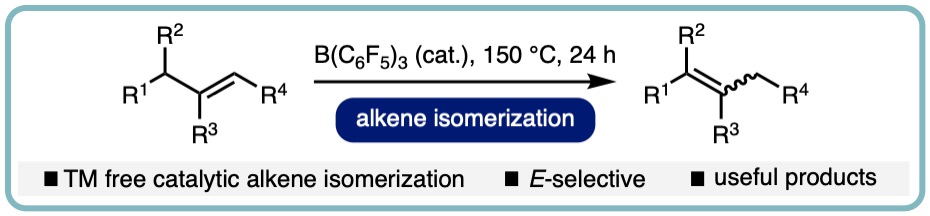
- “B(C6F5)3-Catalyzed (E)-Selective Isomerization of Alkenes”, Betty A. Kustiana, Salma A. Elsherbeni, Thomas G. Linford-Wood, Rebecca L. Melen, Matthew N. Grayson and L. C. Morrill*, Chem. Eur. J., 2022, 28, e202202454. [link]
- “Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes”, S. Basak, L. Winfrey, B. A. Kustiana, R. L. Melen*, L. C. Morrill* and A. P. Pulis*, Chem. Soc. Rev., 2021, 50, 3720-3737. [link]
Conference Contributions:
- 20th Cardiff Chemistry Conference, Cardiff University, 17/05/22, Poster
- 32nd Molecular Synthesis Section Seminar, Cardiff University, 18/03/21, Presentation

Matthew Williams (CDT PhD student July 2019 – March 2023, CDT co-advisors: Dr Duncan Browne and Dr Alastair Lennox)
Ph.D. Examiners: Dr David Nelson (external), Dr Matthew Tredwell (internal)
Next Position: PDRA, RWTH Aachen University, Advisor: Prof. Carsten Bolm
Group spatula: [link]
Publications:
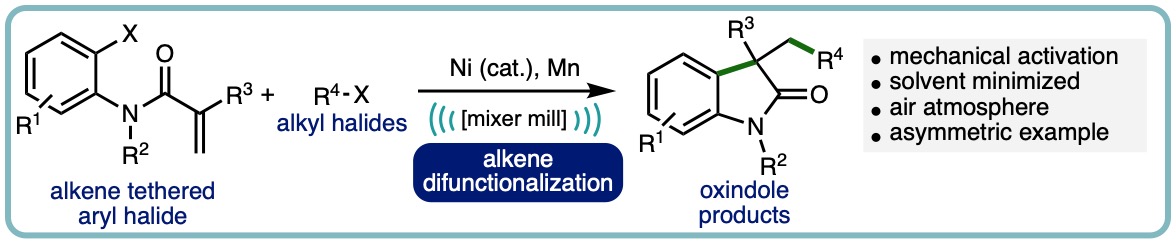
- “Nickel-Catalyzed Intramolecular Alkene Difunctionalization by Ball-Milling”, M. T. J. Williams, L. C. Morrill* and D. L. Browne*, Adv. Synth. Catal., 2023, 365, 1477-1484. [link] [Hot Topic: Earth-Abundant Transition Metal Catalysis]
- “Mechanical Activation of Zero-Valent Metal Reductants for Nickel-Catalyzed Cross-Electrophile Coupling”, Andrew C. Jones†, Matthew, T. J. Williams†, Louis C. Morrill*, and Duncan L. Browne*, ACS Catal., 2022, 12, 13681-13689. [link]
- “Mechanochemical Organocatalysis: Do High Enantioselectivities Contradict what we Might Expect?”, M. T. J. Williams, L. C. Morrill* and D. L. Browne*, ChemSusChem, 2022, 15, e202102157. [link]
- “Expedient Organocatalytic Aza-Morita-Baylis-Hillman Reaction Through Ball-Milling“, M. T. J. Williams, L. C. Morrill* and D. L. Browne*, ACS Sustainable Chem. Eng., 2020, 8, 17876-17881. [link]
- “Synthesis and Reactivity of N-Allenyl Cyanamides”, J. N. Ayres, M. T. J. Williams, G. J. Tizzard, S. J. Coles, K. B. Ling and L. C. Morrill*, Org. Lett., 2018, 20, 5282-5285. [link]
Conference Contributions:
- 20th Cardiff Chemistry Conference, Cardiff University, 17/05/22, Presentation
- 32nd FCG Postgraduate Symposium, Online, 13/05/22, Poster
- RSC Organic Division Poster Symposium, Online, 06/12/21, Poster
- GSK Synthetic Organic Chemistry Postgraduate Symposium, Online, 14/10/21, Presentation and Poster
- 31st Molecular Synthesis Section Seminar, Cardiff University, 14/01/21, Presentation
- Cardiff Catalysis Institute Conference, Cardiff University, 13/01/21, Presentation

Alex Seastram (PhD student Apr 2018 – Oct 2022)
Ph.D. Examiners: Dr Alastair Lennox (external), Prof. Rebecca Melen (internal)
Next Position: Scientist, CatSci, Cardiff, UK.
Group spatula: [link]
Publications:
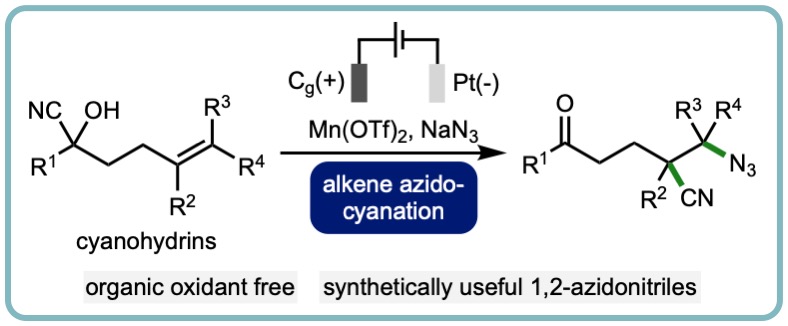
- “Electrochemical Alkene Azidocyanation via 1,4-Nitrile Migration”, Alex C. Seastram, Mishra Deepak Hareram, Thomas M. B. Knight and L. C. Morrill*, Chem. Commun., 2022, 58, 8658-8662. [link]
- “Electrochemical Deconstructive Functionalization of Cycloalkanols via Alkoxy Radicals Enabled by Proton-Coupled Electron Transfer”, Mishra Deepak Hareram, Albara A. M. A. El Gehani, James Harnedy, Alex C. Seastram, Andrew C. Jones, Matthew Burns, Thomas Wirth, Duncan L. Browne and L. C. Morrill*, Org. Lett, 2022, 24, 3890-3895. [link]
- “Ball Milling Enabled Reactivity of Manganese Metal“, W. I. Nicholson, J. L. Howard, G. Magri, A. C. Seastram, A. Khan, R. R. A. Bolt, L. C. Morrill, E. Richards and D. L. Browne*, Angew. Chem. Int. Ed., 2021, 60, 23128-23133 [link]
- “N-Heterocyclic Carbene Acyl Anion Organocatalysis by Ball-Milling“, W. I. Nicholson, A. C. Seastram, S. A. Iqbal, B. G. Reed-Berendt, L. C. Morrill* and D. L. Browne*, ChemSusChem, 2019, 13, 131-135. [link] [Hot Topic: Organocatalysis]
- “Manganese-Catalyzed Electrochemical Deconstructive Chlorination of Cycloalkanols via Alkoxy Radicals”, B. D. W. Allen,† M. D. Hareram,† A. C. Seastram, T. McBride, T. Wirth, D. L. Browne* and L. C. Morrill*, Org. Lett., 2019, 21, 9241-9246. [link] [Associated correction to citations] [link]
Conference Contributions:
- 19th Cardiff Chemistry Conference, 27/10/21, Poster
- GSK Synthetic Organic Chemistry Postgraduate Symposium, 13/10/21, Poster
- 36th RSC Heterocyclic and Synthesis Group Postgraduate Symposium, 07/10/21, Presentation
- 31st SCI’s Fine Chemicals Group Postgraduate Symposium, 06/05/21, Presentation
- 28th Molecular Synthesis Section Seminar, Cardiff University, 16/07/20, Presentation
- CCI Conference, Cardiff University, 15/01/20, Poster

Mubarak Dambatta (Tetfund PhD student Apr 2018 – Oct 2022)
Ph.D. Examiners: Dr James Taylor (external), Prof. Duncan Wass (internal)
Next Position: Scientist, Pharmaron, Cardiff, UK
Group spatula: [link]
Publications:
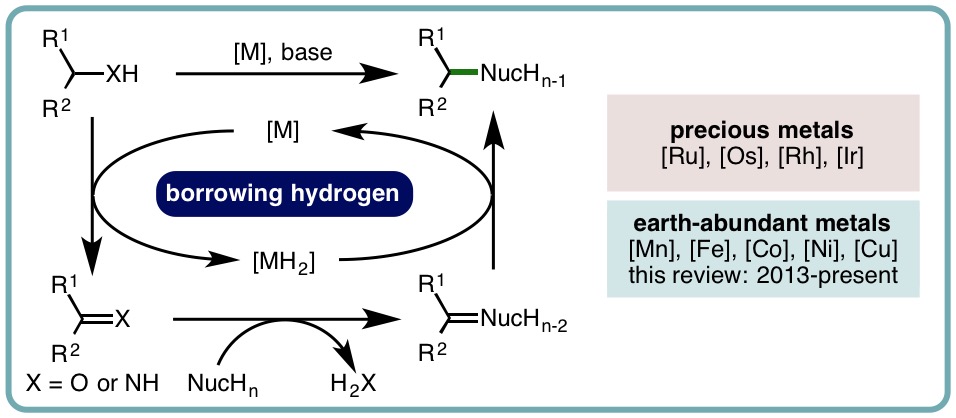
- “Borrowing Hydrogen for Organic Synthesis”, B. G. Reed-Berendt,† D. E. Latham,† M. B. Dambatta and L. C. Morrill*, ACS Cent. Sci., 2021, 7, 570-585. [link]
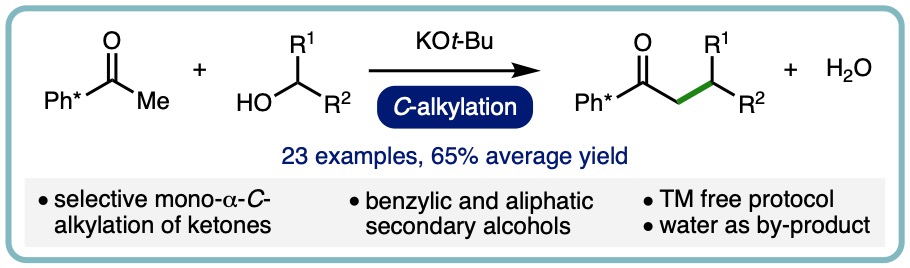
- “Transition Metal Free α-C-Alkylation of Ketones Using Secondary Alcohols“, M. B. Dambatta, J. Santos, R. R. A. Bolt and L. C. Morrill*, Tetrahedron, 2020, 76, 131571. [link] [Invited contribution to the Special Issue on Strategies for Efficient Organic Synthesis Dedicated to the Achievements of Prof. Jonathan Williams]
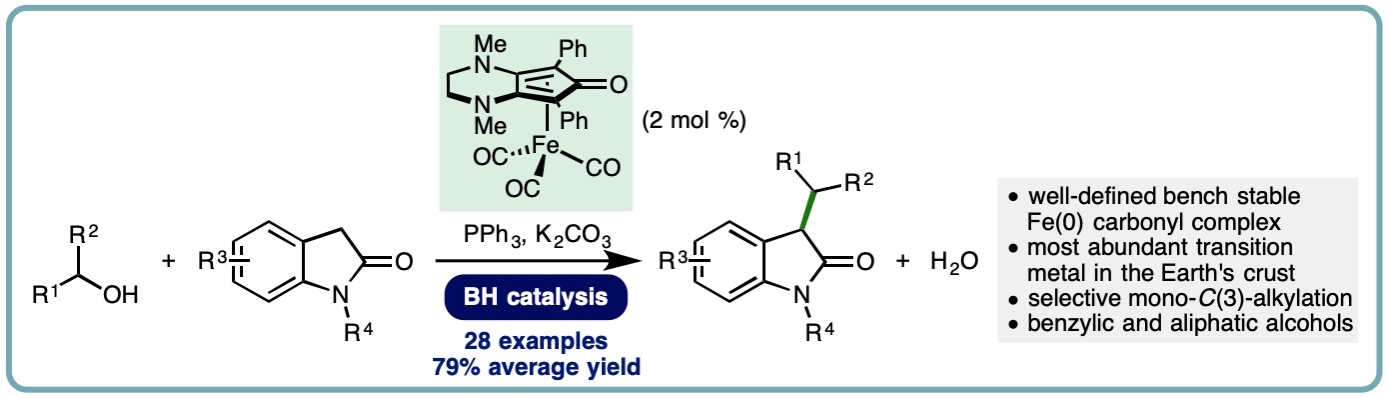
- “Iron-Catalyzed Borrowing Hydrogen C-Alkylation of Oxindoles Using Alcohols“, M. B. Dambatta, K. Polidano, A. D. Northey, J. M. J. Williams and L. C. Morrill*, ChemSusChem, 2019, 12, 2345-2349. [link]
Conference Contributions:
- 22nd Molecular Synthesis Section Seminar, Cardiff University, 11/07/19, Presentation

Daniel Latham (CDT PhD student July 2018 – May 2022)
Ph.D. Examiners: Prof. Martin Wills (external), Dr Paul Newman (internal)
Next Position: Scientist, Sygnature Discovery, Nottingham, UK.
Group spatula: [link]
Publications:
- “Borrowing Hydrogen for Organic Synthesis”, B. G. Reed-Berendt,† D. E. Latham,† M. B. Dambatta and L. C. Morrill*, ACS Cent. Sci., 2021, 7, 570-585. [link]
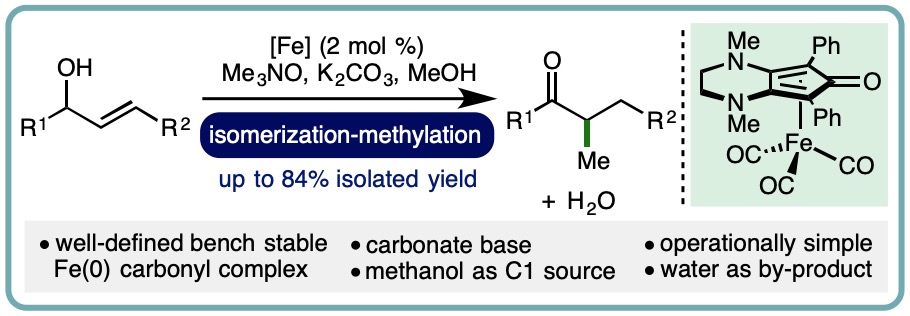
- “One-Pot Conversion of Allylic Alcohols to α-Methyl Ketones via Iron-Catalyzed Isomerization-Methylation“, D. E. Latham, K. Polidano, J. M. J. Williams and L. C. Morrill*, Org. Lett., 2019, 21, 7914-7918. [link]
Conference Contributions:
- 19th Cardiff Chemistry Conference, 27/10/21, Poster
- 26th Molecular Synthesis Section Seminar, Cardiff University, 12/03/20, Presentation
- CCI Conference, Cardiff University, 15/01/20, Poster
- EPSRC CDT Catalysis Spring Conference, Cardiff University, 06/06/19, Presentation and Poster

Deepak Mishra (PhD student Oct 2018 – Sept 2021)
Ph.D. Examiners: Dr Benjamin Buckley (external), Dr Niek Buurma (internal)
Next position: PDRA, The University of Manchester, Advisor: Prof. Igor Larrosa
Group spatula: [link]
Publications:
- “Electrochemical Alkene Azidocyanation via 1,4-Nitrile Migration”, Alex C. Seastram, Mishra Deepak Hareram, Thomas M. B. Knight and L. C. Morrill*, Chem. Commun., 2022, 58, 8658-8662. [link]
- “Electrochemical Deconstructive Functionalization of Cycloalkanols via Alkoxy Radicals Enabled by Proton-Coupled Electron Transfer”, Mishra Deepak Hareram, Albara A. M. A. El Gehani, James Harnedy, Alex C. Seastram, Andrew C. Jones, Matthew Burns, Thomas Wirth, Duncan L. Browne and L. C. Morrill*, Org. Lett, 2022, 24, 3890-3895. [link]
- “Electrochemical oxidative Z-selective C(sp2)-H chlorination of acrylamides”, J. Harnedy, M. D. Hareram, G. J. Tizzard, S. J. Coles and L. C. Morrill*, Chem. Commun., 2021, 57, 12643-12646. [link] [Invited contribution to the 2021 Emerging Investigators themed collection]
- “Manganese-Catalyzed Electrochemical Deconstructive Chlorination of Cycloalkanols via Alkoxy Radicals”, B. D. W. Allen,† M. D. Hareram,† A. C. Seastram, T. McBride, T. Wirth, D. L. Browne* and L. C. Morrill*, Org. Lett., 2019, 21, 9241-9246. [link] [Associated correction to citations] [link]
Conference Contributions:
- 19th Cardiff Chemistry Conference, 27/10/21, Poster
- School Seminar, Cardiff University, 29/03/21, Presentation
- 27th Molecular Synthesis Section Seminar, Cardiff University, 28/05/20, Presentation
- CCI Conference, Cardiff University, 15/01/20, Poster

Benjamin Reed-Berendt (PhD student Oct 2016 – Mar 2020)
Ph.D. Examiners: Dr Stephen Thomas (external), Dr Ian Fallis (internal)
Next position: Process Development Chemist, CatSci, Cardiff, UK
Group spatula: [link]
Publications:
- “Borrowing Hydrogen for Organic Synthesis”, B. G. Reed-Berendt,† D. E. Latham,† M. B. Dambatta and L. C. Morrill*, ACS Cent. Sci., 2021, 7, 570-585. [link]
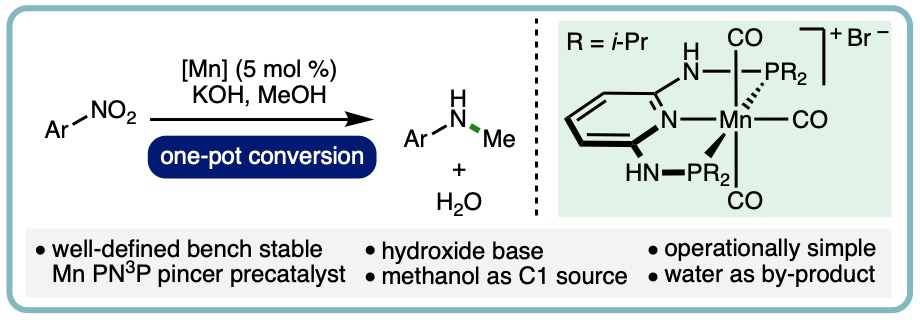
- “Manganese-Catalyzed One-Pot Conversion of Nitroarenes into N-Methylarylamines Using Methanol“, B. G. Reed-Berendt, N. Mast and L. C. Morrill*, Eur. J. Org. Chem., 2020, 1136-1140. [link] [Invited contribution to the YourJOC Talents Special Issue]
- “N-Heterocyclic Carbene Acyl Anion Organocatalysis by Ball-Milling“, W. I. Nicholson, A. C. Seastram, S. A. Iqbal, B. G. Reed-Berendt, L. C. Morrill* and D. L. Browne*, ChemSusChem, 2019, 13, 131-135. [link] [Hot Topic: Organocatalysis]
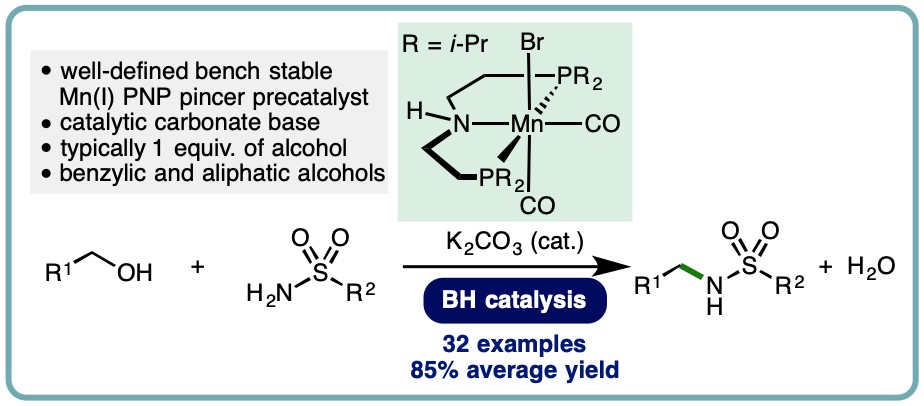
- “Manganese-Catalyzed N-Alkylation of Sulfonamides Using Alcohols“, B. G. Reed-Berendt and L. C. Morrill*, J. Org. Chem., 2019, 84, 3715-3724. [link] [Highlighted in Organic Chemistry Portal] [link]
- “Recent Advances in Homogeneous Borrowing Hydrogen Catalysis using Earth-Abundant First Row Transition Metals”, B. G. Reed-Berendt, K. Polidano and L. C. Morrill*, Org. Biomol. Chem., 2019, 17, 1595-1607. [link] [Invited contribution to the New Talent Special Issue]
- “FLP-Catalyzed Transfer Hydrogenation of Silyl Enol Ethers”, I. Khan, B. G. Reed-Berendt, R. L. Melen* and L. C. Morrill*, Angew. Chem. Int. Ed., 2018, 57, 12356-12359. [link]
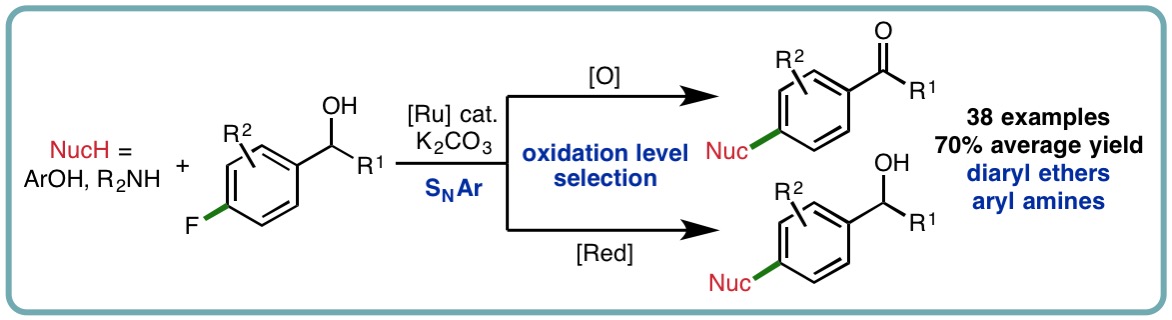
- “Exploring Tandem Ruthenium-Catalyzed Hydrogen Transfer and SNAr Chemistry”, K. Polidano, B. G. Reed-Berendt, A. Basset, A. J. A. Watson, J. M. J. Williams and L. C. Morrill*, Org. Lett., 2017, 19, 6716-6719. [link]
Conference Contributions:
- 18th Annual Cardiff Chemistry Conference, Cardiff University, 15/05/19, Presentation
- RSC Organic Division South-West Regional Meeting, Cardiff University, 24/01/19, Poster
- 13th Molecular Synthesis Section Seminar, Cardiff University, 18/01/18, Presentation
- RSC Organic Division South-West Regional Meeting, University of Bristol, 17/01/18, Poster

Kurt Polidano (CDT PhD student since Oct 2016 – Oct 2019, CDT co-advisor: Prof. Jonathan Williams)
Ph.D. Examiners: Prof Steve Marsden (external), Dr Ben Ward (internal)
Next position: Process Development Chemist, CatSci, Cardiff, UK
Group spatula: [link]
Publications:
- “One-Pot Conversion of Allylic Alcohols to α-Methyl Ketones via Iron-Catalyzed Isomerization-Methylation“, D. E. Latham, K. Polidano, J. M. J. Williams and L. C. Morrill*, Org. Lett., 2019, 21, 7914-7918. [link]
- “Iron-Catalyzed Borrowing Hydrogen β-C(sp3)-Methylation of Alcohols“, K. Polidano, J. M. J. Williams and L. C. Morrill*, ACS Catal., 2019, 9, 8575-8580. [link] [Highlighted in Synfacts, 2019, 15, 1280] [link]
- “Iron-Catalyzed Borrowing Hydrogen C-Alkylation of Oxindoles Using Alcohols“, M. B. Dambatta, K. Polidano, A. D. Northey, J. M. J. Williams and L. C. Morrill*, ChemSusChem, 2019, 12, 2345-2349. [link]
- “Recent Advances in Homogeneous Borrowing Hydrogen Catalysis using Earth-Abundant First Row Transition Metals”, B. G. Reed-Berendt, K. Polidano and L. C. Morrill*, Org. Biomol. Chem., 2019, 17, 1595-1607. [link] [Invited contribution to the New Talent Special Issue]
- “Iron-Catalyzed Methylation using the Borrowing Hydrogen Approach”, K. Polidano, B. D. W. Allen, J. M. J. Williams and L. C. Morrill*, ACS Catal., 2018, 8, 6440-6445. [link]
- “Exploring Tandem Ruthenium-Catalyzed Hydrogen Transfer and SNAr Chemistry”, K. Polidano, B. G. Reed-Berendt, A. Basset, A. J. A. Watson, J. M. J. Williams and L. C. Morrill*, Org. Lett., 2017, 19, 6716-6719. [link]
Conference Contributions:
- EPSRC CDT Catalysis Spring Conference, Cardiff University, 06/06/19, Presentation and Poster
- RSC Organic Division South-West Regional Meeting, Cardiff University, 24/01/19, Poster
- 14th Molecular Synthesis Section Seminar, Cardiff University, 15/03/18, Presentation
- RSC Organic Division South-West Regional Meeting, University of Bristol, 17/01/18, Poster
- EPSRC CDT Catalysis Spring Conference, Cardiff University, 06/06/17, Presentation and Poster

James Ayres (PhD student, Oct 2015 – Sept 2018)
Ph.D. Examiners: Dr Marc Kimber (external), Prof. Thomas Wirth (internal)
Next position: PDRA, The University of Leeds, Advisor: Dr Richard Foster
Group spatula: [link]
Publications:
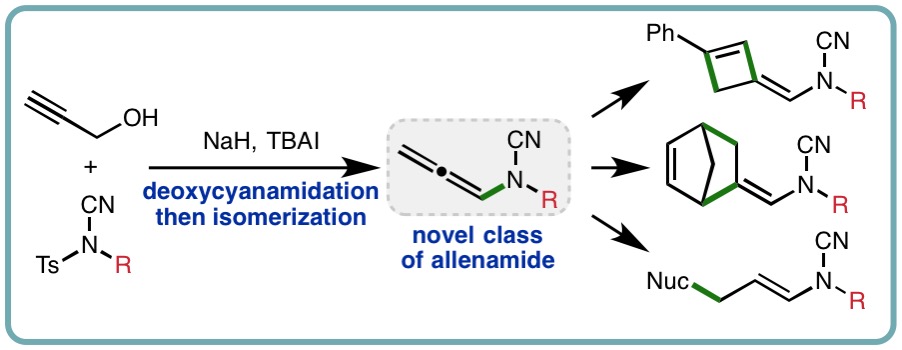
- “Synthesis and Reactivity of N-Allenyl Cyanamides”, J. N. Ayres, M. T. J. Williams, G. J. Tizzard, S. J. Coles, K. B. Ling and L. C. Morrill*, Org. Lett., 2018, 20, 5282-5285. [link]
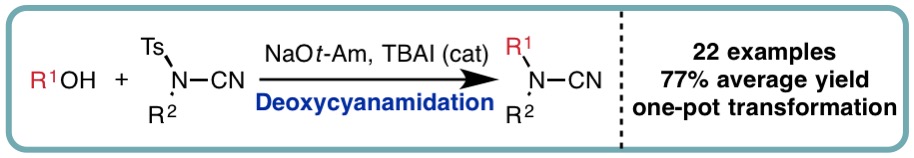
- “Deoxycyanamidation of Alcohols with N-Cyano-N-Phenyl-p-Methylbenzenesulfonamide (NCTS)”, J. N. Ayres, M. W. Ashford, Y. Stöckl, V. Prudhomme, K. B. Ling, J. A. Platts and L. C. Morrill*, Org. Lett., 2017, 19, 3835-3838. [link]
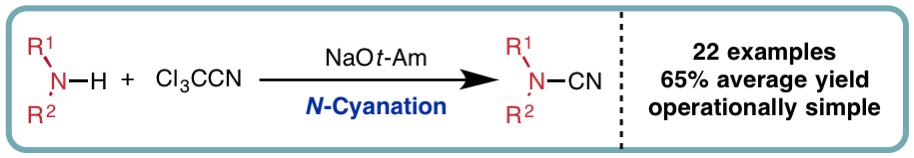
- “N-Cyanation of Secondary Amines using Trichloroacetonitrile”, J. N. Ayres, K. B. Ling* and L. C. Morrill*, Org. Lett., 2016, 18, 5528-5531. [link]
Conference Contributions:
- RSC Organic Division South-West Regional Meeting, University of Bristol, 17/01/18, Poster
- Departmental Seminar, Indian Institute of Chemical Technology (IICT), Hyderabad, India, 22/11/17, Presentation
- J-NOST-13 Conference, Banaras Hindu University, Lucknow University, India, 09/11/17, Presentation
- 32nd Postgraduate Symposium of the RSC Heterocyclic and Synthesis Group, London, 20/09/17, Poster and Presentation
- 10th Molecular Synthesis Section Seminar, Cardiff University, 13/07/17, Session Chair
- Bio-Techne Chemistry Poster Symposium, Bristol, 25/05/17, Poster
- 16th Cardiff Chemistry Conference, Cardiff University, 15/05/17, Poster
- 8th Molecular Synthesis Section Seminar, Cardiff University, 16/03/17, Presentation
- RSC Organic Division South-West Regional Meeting, University of Bath, 01/02/17, Poster
Co-Supervised PhD Students

Andrew Jones (KESS 2 PhD student Oct 2018 – Mar 2022, Collaborative project led by Dr Duncan Browne)
Ph.D. Examiners: Dr Ai-Lan Lee (external), Prof. Thomas Wirth (internal)
Next position: Scientist, Domainex, Cambridge, UK.
Publications:
- “Mechanical Activation of Zero-Valent Metal Reductants for Nickel-Catalyzed Cross-Electrophile Coupling”, Andrew C. Jones†, Matthew, T. J. Williams†, Louis C. Morrill*, and Duncan L. Browne*, ACS Catal., 2022, 12, 13681-13689. [link]
- “Electrochemical Deconstructive Functionalization of Cycloalkanols via Alkoxy Radicals Enabled by Proton-Coupled Electron Transfer”, Mishra Deepak Hareram, Albara A. M. A. El Gehani, James Harnedy, Alex C. Seastram, Andrew C. Jones, Matthew Burns, Thomas Wirth, Duncan L. Browne and L. C. Morrill*, Org. Lett, 2022, 24, 3890-3895. [link]
Conference Contributions:
- 19th Cardiff Chemistry Conference, 27/10/21, Poster
- 27th Molecular Synthesis Section Seminar, Cardiff University, 28/05/20, Presentation
- CCI Conference, Cardiff University, 15/01/20, Poster
- Enabling Technologies for Synthesis Workshop, Cardiff University, 28/06/19, Poster
CDT Research Sabbatical Students

Taniya Shandil (Catalysis CDT research sabbatical 1 student, CDT co-advisor: Prof. Jonathan Williams, Jun-Sept 2016)

Mattia Manzotti (Catalysis CDT research sabbatical 1 student, Collaborative project led by Dr Rebecca Melen, Jan-May 2016)
Next position: PhD, Bristol University, Advisor: Prof. Robin Bedford
Publications:
- “Frustrated Lewis Pair (FLP)-Catalyzed Hydrogenation of Aza-Morita-Baylis-Hillman Adducts and Sequential Organo-FLP Catalysis”, I. Khan, M. Manzotti, G. J. Tizzard, S. J. Coles, R. L. Melen* and L. C. Morrill*, ACS Catal., 2017, 7, 7748-7752. [link]

Jonathan Hall (Catalysis CDT research sabbatical 1 student, Collaborative project led by Dr Louis Luk, Jan-May 2016)
Next position: PhD, Bath University, Advisor: Prof. Mike Whittlesey
Publications:
- “Reactivity and Selectivity of Iminium Organocatalysis Improved by a Protein Host”, A. R. Nödling, K. Swiderek, R. Castillo, J. W. Hall, A. Angelastro, L. C. Morrill, Y. Jin, Y-H. Tsai, V. Moliner* and L. Y. P. Luk*, Angew. Chem. Int. Ed., 2018, 57, 12478-12482. [link]
Postgraduate Project Students

Jamie Hall (MSc medicinal chemistry postgraduate researcher, Jun – Aug 2023)

Gwen Weldon (MSc advanced chemistry postgraduate researcher, Jun – Aug 2023)

Alex Maguire (MSc advanced chemistry postgraduate researcher, Jun – Aug 2022)

Adam Gage (MSc advanced chemistry postgraduate researcher, Jun – Aug 2021)

Lia Mitchell (MSc medicinal chemistry postgraduate researcher, Jun – Aug 2021)

Seán O’Rourke (MSc medicinal chemistry postgraduate researcher, Jun – Aug 2019)
Undergraduate Project Students (MChem)

Ethan Herneman (MChem student, Oct 2022 – May 2023)

Emily Harper (MChem student, Oct 2022 – May 2023)

Luke Shepherd (MChem student, Oct 2021- May 2022)
Next Position: Graduate Scientist at Autifony Therapeutics
Tom Knight (M.Chem student, Oct 2020 – May 2021)
Next position: PhD, UCL, Advisor: Prof. Matthew Todd
Publications:
- “Electrochemical Alkene Azidocyanation via 1,4-Nitrile Migration”, Alex C. Seastram, Mishra Deepak Hareram, Thomas M. B. Knight and L. C. Morrill*, Chem. Commun., 2022, 58, 8658-8662. [link]

Luke Ward (M.Chem student, Oct 2020 – May 2021)
Next position: PhD, University of Bristol, Advisor: Dr Ali Lennox

Jake Painter (M.Chem student, Oct 2020 – May 2021)
Next position: PhD, University of Liverpool, Advisor: Prof. John Bower

Joe Santos (MChem project student, Oct 2019 – May 2020)
Publications:
- “Transition Metal Free α-C-Alkylation of Ketones Using Secondary Alcohols“, M. B. Dambatta, J. Santos, R. R. A. Bolt and L. C. Morrill*, Tetrahedron, 2020, 76, 131571. [link] [Invited contribution to the Special Issue on Strategies for Efficient Organic Synthesis Dedicated to the Achievements of Prof. Jonathan Williams]
Mark Shuttleworth (MChem project student, Oct 2019 – May 2020)
Next position: PhD, University of Bath, Advisor: Dr James Taylor

Ioan Bale (MChem project student, Oct 2019 – May 2020)

Alex Bardsley (MChem project student, Oct 2018 – May 2019)

Callum Adams (MChem project student, Oct 2018 – May 2019)
Next position: PhD, University of St Andrews, Advisor: Dr Craig Johnston

Beth Grattidge (MChem project student, Oct 2018 – May 2019)

Alex Seastram (MChem project student, Oct 2017 – May 2018)
Prizes: Outstanding 4th year MChem Performance
Next position: PhD, Cardiff University, Advisor: Dr Louis C. Morrill
Publications:
- “N-Heterocyclic Carbene Acyl Anion Organocatalysis by Ball-Milling“, W. I. Nicholson, A. C. Seastram, S. A. Iqbal, B. G. Reed-Berendt, L. C. Morrill* and D. L. Browne*, ChemSusChem, 2019, 13, 131-135. [link] [Hot Topic: Organocatalysis]

Matthew Williams (MChem project student, Oct 2017 – May 2018)
Prizes: GSK prize for best MChem project in molecular synthesis section
Next position: PhD, Cardiff University, Advisor: Dr Louis C. Morrill, CDT co-advisors: Dr Duncan Browne and Dr Alastair Lennox
Publications:
- “Synthesis and Reactivity of N-Allenyl Cyanamides”, J. N. Ayres, M. T. J. Williams, G. J. Tizzard, S. J. Coles, K. B. Ling and L. C. Morrill*, Org. Lett., 2018, 20, 5282-5285. [link]

Publications:
- “Iron-Catalyzed Borrowing Hydrogen C-Alkylation of Oxindoles Using Alcohols“, M. B. Dambatta, K. Polidano, A. D. Northey, J. M. J. Williams and L. C. Morrill*, ChemSusChem, 2019, 12, 2345-2349. [link]
Alex Northey (MChem project student, Oct 2017 – May 2018)

Matthew Ashford (MChem project student, Oct 2016 – May 2017)
Next position: PhD, University of St Andrews, Advisor: Dr Allan Watson
Publications:
- “Deoxycyanamidation of Alcohols with N-Cyano-N-Phenyl-p-Methylbenzenesulfonamide (NCTS)”, J. N. Ayres, M. W. Ashford, Y. Stöckl, V. Prudhomme, K. B. Ling, J. A. Platts and L. C. Morrill*, Org. Lett., 2017, 19, 3835-3838. [link]

Daniel Moseley (MChem project student, Oct 2016 – May 2017)
Next position: PhD, Oxford Synthesis for Biology & Medicine CDT, Advisor: Prof. Michael Willis

Bryna Harris (MChem project student, Oct 2016 – May 2017)

Max Smith (MChem project student, Oct 2016 – May 2017)

Benjamin Reed- Berendt (MChem project student, Oct 2015 – May 2016)
Next position: PhD, Cardiff University, Advisor: Dr Louis C. Morrill

Matthew Jenner (MChem project student, Oct 2015 – May 2016)
Next position: PhD, University of Southampton, Advisor: Dr Ramon Rios

Tyler Woods (MChem project student, Oct 2015 – May 2016)
Undergraduate Project Students (BSc)

Luis Cleaton (BSc project student, Jan-May 2023)

Gwen Weldon (BSc project student, Jan-May 2022)

Adam Gage (BSc project student, Jan-May 2020)

Sam Parker (BSc project student, Jan-May 2018)

Connor Radcliffe (BSc project student, Jan-May 2018)

Thomas Lambourn (BSc project student, Jan-May 2017)

Niall Hope (BSc project student, Jan-May 2016)
Undergraduate Project Students (Cardiff Internship)

Ted Gushlow (CUROP undergraduate researcher, Jun-Aug 2023)
Research Area: Borrowing hydrogen catalysis

Abbie Harris (CUROP undergraduate researcher, July-Aug 2022)
Research Area: Borrowing hydrogen catalysis

Daniel Gorst (CUROP undergraduate researcher, May-Aug 2022)
Research Area: Synthetic Organic Electrochemistry

Robert Bolt (CUROP undergraduate researcher, Jun-Aug 2019)
Next position: MChem, Cardiff University
Publications:
- “Transition Metal Free α-C-Alkylation of Ketones Using Secondary Alcohols“, M. B. Dambatta, J. Santos, R. R. A. Bolt and L. C. Morrill*, Tetrahedron, 2020, 76, 131571. [link] [Invited contribution to the Special Issue on Strategies for Efficient Organic Synthesis Dedicated to the Achievements of Prof. Jonathan Williams]

Joe Santos (CUROP undergraduate researcher, Aug-Sept 2018)
Next position: MChem, Cardiff University

Luke Ward (CUROP undergraduate researcher, Aug-Sept 2018)
Next position: MChem, Cardiff University

Ashley Ramdass (CUROP undergraduate researcher, Jun-Aug 2018)
Next position: MChem, Cardiff University

Thomas Knight (CUROP undergraduate researcher, Jun-Aug 2018)
Next position: MChem, Cardiff University

Alexi Sedikides (Internship student, Jun-Sept 2016)
Next position: MChem, Cardiff University

Vladimir Vladimirov (RSC undergraduate bursary scholar, Collaborative project led by Dr Rebecca Melen, Jun-Jul 2016)
Next position: MChem, Cardiff University
Undergraduate Project Students (Visiting Scholars)

Nicolas Mast (ERASMUS internship student May-Aug 2019)
Next position: MEng in Chemistry, ENSCR
Publications:
- “Manganese-Catalyzed One-Pot Conversion of Nitroarenes into N-Methylarylamines Using Methanol“, B. G. Reed-Berendt, N. Mast and L. C. Morrill*, Eur. J. Org. Chem., 2020, 1136-1140. [link] [Invited contribution to the YourJOC Talents Special Issue]

Marcus Espe (ERASMUS project student, since Sept-Dec 2017)
Next position: MSc in Chemistry, Aarhus University

Anaïs Basset (ERASMUS internship student, Jan-May 2017)
Next position: MA in Chemistry, ENSCM
Publications:
- “Exploring Tandem Ruthenium-Catalyzed Hydrogen Transfer and SNAr Chemistry”, K. Polidano, B. G. Reed-Berendt, A. Basset, A. J. A. Watson, J. M. J. Williams and L. C. Morrill*, Org. Lett., 2017, 19, 6716-6719. [link]

Yannick Stöckl (RISE Worldwide internship student, Aug-Oct 2016)
Next position: MSc in Chemistry, University of Stuttgart
Publications:
- “Deoxycyanamidation of Alcohols with N-Cyano-N-Phenyl-p-Methylbenzenesulfonamide (NCTS)”, J. N. Ayres, M. W. Ashford, Y. Stöckl, V. Prudhomme, K. B. Ling, J. A. Platts and L. C. Morrill*, Org. Lett., 2017, 19, 3835-3838. [link]

Ng Yoke Yin (Internship student, Sept-Oct 2016)
Next position: Diploma in Medicinal Chemistry, Nangyang Polytechnic

Anissa Naïr (ERASMUS internship student, Jun-Aug 2016)
Next position: MA in Chemistry, ENSCCF

Vassili Prudhomme (ERASMUS internship student, Jun-Aug 2016)
Next position: MA in Chemistry, ENSCCF
Publications:
- “Deoxycyanamidation of Alcohols with N-Cyano-N-Phenyl-p-Methylbenzenesulfonamide (NCTS)”, J. N. Ayres, M. W. Ashford, Y. Stöckl, V. Prudhomme, K. B. Ling, J. A. Platts and L. C. Morrill*, Org. Lett., 2017, 19, 3835-3838. [link]