Clinical Trials As A Catalyst For Change In The ‘Big Easy’ – The 40th Society For Clinical Trials Annual Meeting, New Orleans, USA
13 June 2019
By Victoria Shepherd and Nigel Kirby
A taste of New Orleans
At times New Orleans felt very much like Wales…… wet. However, when the sun came out and the puddles dried up New Orleans was a place full of culture and friendly faces. The French Quarter is a vibrant place to visit in the day or night. This is full of a whatever type of food you could wish to eat and filled with little market shops and stalls along with their cast iron balconies on the side of buildings. Jazz is certainly the sound of New Orleans with plenty of jazz cafes / bars and street performers to be able to give you a taste of New Orleans. From their jazzy soundtrack and tropical climate, this is a destination everyone can enjoy. Which we did!

‘Clinical Trials: a catalyst for societal advancement through innovation’
The Society for Clinical Trials annual meeting ran for three days in May, with a number of pre-conference and in-conference workshops available. The theme of the meeting as ‘a catalyst’ was intended to unify the diverse professions, disciplines, organisations, and areas of health that were brought together to share experiences of designing, conducting and analysing clinical trials, all in the name of changing lives through improving health. The programme reflected this through offering sessions on a wide range of topics on clinical trials conduct and methodology, with the aim of developing and disseminating optimal methods and best practices in clinical trials around the world.
The Centre for Trials Research delegation
Some of the Centre’s work featured in three presentations at the conference. Nigel Kirby presented two studies in his presentation ‘‘First do no harm’ – exploring the potential impact of placebo treatments on adverse effects’. In the ‘Ethics’ session he presented the findings from a systematic review of nocebo effects reported by participants receiving placebo in clinical trials (paper published in Trials) and a content analysis of information provided on adverse effects in Participant Information Sheets (paper to be submitted shortly).

In the same session Victoria Shepherd presented a systematic review of the ethical aspects of surrogate decision-making for clinical trials involving adults who lack capacity to consent (paper published in AJOB Empirical Bioethics). She also presented the findings of the DECISION Study which explored family members’ experiences of acting as a consultee or legal representative on behalf of someone who lacked capacity to consent.
Our highlights from the conference
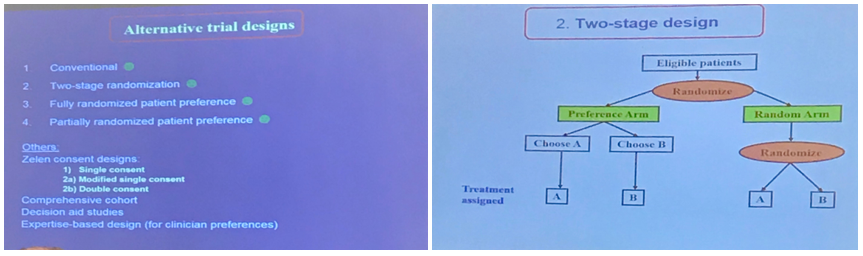
The programme was extensive, and included sessions on trial design and management, recruitment and retention, outcomes, statistical analysis, and regulation and consent. Some of our highlights included a fascinating workshop on ‘patient preference trial designs’ which are designed to include patient choice (e.g to be randomised or not, or choice of trial arm), sometimes randomised through two or more stages. This enables trials to take account of patient preference effects as well as treatment effects.
Data Management and IS systems are popular topics at this conference and the ways that others are collecting and managing data allows for great ideas for what we can look to implement here in the Centre. This includes participant diary data being collected entirely by text message with the text messages linking to other answers that participants have given so they are not having to see unnecessary questions. Another presentation covered how well do we know our sites and what key metrics we can use to monitor them.

The conference next year is in Baltimore (known as Charm City) with the theme of ‘Enhancing and Enabling the Clinical Trials Ecosystem through Interdisciplinary Collaborations’. Something to keep in mind when dissemination planning!
- April 2024
- March 2024
- December 2023
- November 2023
- September 2023
- July 2023
- June 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- November 2021
- September 2021
- July 2021
- June 2021
- May 2021
- March 2021
- February 2021
- December 2020
- November 2020
- September 2020
- August 2020
- July 2020
- January 2020
- December 2019
- October 2019
- September 2019
- July 2019
- June 2019
- May 2019
- April 2019
- February 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- December 2017
- October 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- October 2016
- August 2016
- June 2016
- April 2016
- March 2016
- February 2016